Volume 18, Issue 3 (May-Jun 2024)
mljgoums 2024, 18(3): 4-7 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Devhare D, Pol S. Clinical and microbiological study of vancomycin-resistant enterococci isolated from colonized and infected patients with special reference to risk factors. mljgoums 2024; 18 (3) :4-7
URL: http://mlj.goums.ac.ir/article-1-1680-en.html
URL: http://mlj.goums.ac.ir/article-1-1680-en.html
1- BVDUMC, Pune, India , deepadevhare@gmail.com
2- BJ. Government Medical College, Pune, India
2- BJ. Government Medical College, Pune, India
Full-Text [PDF 391 kb]
(1151 Downloads)
| Abstract (HTML) (4749 Views)
Full-Text: (930 Views)
Introduction
Enterococcus is a typical commensal bacterial genus of humans' oral cavity, gut, and genitourinary tract (1,2). However, it has become a growing concern in healthcare settings as they are a significant cause of many nosocomial infections worldwide (3-5). Some species of the genus Enterococcus, such as Enterococcus faecium and Enterococcus faecalis, are important opportunistic pathogens that can cause severe infections in susceptible hosts (2,6). This organism can survive in the hospital environment longer due to its intrinsic resistance to several commonly used antibiotics and its ability to acquire resistance to all currently available antibiotics.
The increasing and inappropriate use of antibiotics, such as cephalosporins and especially vancomycin, has led to the emergence and increase in the prevalence of colonization and infections by vancomycin-resistant enterococci (VRE) (7). Other underlying clinical conditions and known risk factors can predispose to enterococcal colonization and infection, such as structural abnormality of the urinary tract, hematologic malignancy, neutropenia, hepatorenal insufficiency, hypoalbuminemia, diabetes mellitus, prolonged hospital stay, and use of immunosuppressants (8-12). The infections associated with VRE are urinary tract infections, endocarditis, bacteremia, wound infections, intraperitoneal infections, and meningitis (13-16).
The clinical profile and risk factors for VRE infection and colonization must be studied, as they are poorly understood (6). This helps identify the high-risk patients who have a higher chance of getting an infection with VRE. As vancomycin resistance is plasmid-encoded, precautionary and infection control measures can be taken in advance to prevent VRE infection and its spread in the hospital environment (10,13,17). Therefore, the present study was conducted to study the clinical spectrum of VRE infections, risk factors associated with VRE infection, and colonization and to determine the source of VRE transmission in a tertiary care hospital in western Maharashtra, India.
Methods
A prospective observational study was conducted at the Microbiology Department of a tertiary care hospital in western Maharashtra, India. The study period was from January 2013 to December 2013 and commenced after approval by the institutional ethics committee. (Reference No.- BMC/IEC/Pharmac/D0313005-05). Informed consent was obtained from all patients included in the study.
Patients of all age groups and both sexes visiting the inpatient and outpatient Departments of a tertiary care hospital in western Maharashtra, India. The first group included 200 enterococcal strains isolated from clinical samples of urine, blood, cerebrospinal fluid (CSF), pleural fluid, ascetic fluid, pus, and wound swabs from patients treated at the hospital with different infections. The second group included 200 stool samples from patients without any infections who were admitted to the hospital and screened for the presence of VRE in the gastrointestinal tract.
Details of personal history along with the history of hospitalization, clinical history regarding any underlying comorbidity or predisposing factors such as diabetes mellitus, malignancy, surgical procedure, immunosuppressive drug therapy, any systemic illness, antibiotic treatment history, and duration of the hospital stay of each patient who presented infection and colonization by VRE were obtained.
Blood agar (Himedia, India) and MacConkey agar (Himedia, India) plates were used to inoculate clinical samples. Samples were inoculated on the culture media with a sterile nichrome loop and incubated under aerobic conditions at 37 °C for 24-48 h. Colony morphology, catalase test, and Gram stain were used for preliminary identification of Enterococci. For genus-level identification of Enterococcus, bile aesculin test, 6.5% salt tolerance test, and pyrrolidinyl arylamines (PYR) tests were used. Species-level identification was made only for isolates showing resistance to vancomycin by the Kirby Bauer disc diffusion method. Mannitol fermentation, motility, pigment production, arabinose fermentation, and arginine hydrolysis tests were used for species-level identification (14-16).
Stool samples were screened for the presence of VRE by using bile esculin azide agar plus 6 µg/mL of vancomycin (BEAV, Himedia, India). After 24 h of incubation, if at least one colony had grown along with the darkening of the medium, then Gram stain and catalase tests were performed for presumptive identification of Enterococci (17). The microbiological tests used for species level identification were the same as described above in "Isolation and identification of Enterococcus spp. from clinical samples" (14-16).
Vancomycin susceptibility testing:
Enterococcus is a typical commensal bacterial genus of humans' oral cavity, gut, and genitourinary tract (1,2). However, it has become a growing concern in healthcare settings as they are a significant cause of many nosocomial infections worldwide (3-5). Some species of the genus Enterococcus, such as Enterococcus faecium and Enterococcus faecalis, are important opportunistic pathogens that can cause severe infections in susceptible hosts (2,6). This organism can survive in the hospital environment longer due to its intrinsic resistance to several commonly used antibiotics and its ability to acquire resistance to all currently available antibiotics.
The increasing and inappropriate use of antibiotics, such as cephalosporins and especially vancomycin, has led to the emergence and increase in the prevalence of colonization and infections by vancomycin-resistant enterococci (VRE) (7). Other underlying clinical conditions and known risk factors can predispose to enterococcal colonization and infection, such as structural abnormality of the urinary tract, hematologic malignancy, neutropenia, hepatorenal insufficiency, hypoalbuminemia, diabetes mellitus, prolonged hospital stay, and use of immunosuppressants (8-12). The infections associated with VRE are urinary tract infections, endocarditis, bacteremia, wound infections, intraperitoneal infections, and meningitis (13-16).
The clinical profile and risk factors for VRE infection and colonization must be studied, as they are poorly understood (6). This helps identify the high-risk patients who have a higher chance of getting an infection with VRE. As vancomycin resistance is plasmid-encoded, precautionary and infection control measures can be taken in advance to prevent VRE infection and its spread in the hospital environment (10,13,17). Therefore, the present study was conducted to study the clinical spectrum of VRE infections, risk factors associated with VRE infection, and colonization and to determine the source of VRE transmission in a tertiary care hospital in western Maharashtra, India.
Methods
A prospective observational study was conducted at the Microbiology Department of a tertiary care hospital in western Maharashtra, India. The study period was from January 2013 to December 2013 and commenced after approval by the institutional ethics committee. (Reference No.- BMC/IEC/Pharmac/D0313005-05). Informed consent was obtained from all patients included in the study.
Patients of all age groups and both sexes visiting the inpatient and outpatient Departments of a tertiary care hospital in western Maharashtra, India. The first group included 200 enterococcal strains isolated from clinical samples of urine, blood, cerebrospinal fluid (CSF), pleural fluid, ascetic fluid, pus, and wound swabs from patients treated at the hospital with different infections. The second group included 200 stool samples from patients without any infections who were admitted to the hospital and screened for the presence of VRE in the gastrointestinal tract.
Details of personal history along with the history of hospitalization, clinical history regarding any underlying comorbidity or predisposing factors such as diabetes mellitus, malignancy, surgical procedure, immunosuppressive drug therapy, any systemic illness, antibiotic treatment history, and duration of the hospital stay of each patient who presented infection and colonization by VRE were obtained.
Blood agar (Himedia, India) and MacConkey agar (Himedia, India) plates were used to inoculate clinical samples. Samples were inoculated on the culture media with a sterile nichrome loop and incubated under aerobic conditions at 37 °C for 24-48 h. Colony morphology, catalase test, and Gram stain were used for preliminary identification of Enterococci. For genus-level identification of Enterococcus, bile aesculin test, 6.5% salt tolerance test, and pyrrolidinyl arylamines (PYR) tests were used. Species-level identification was made only for isolates showing resistance to vancomycin by the Kirby Bauer disc diffusion method. Mannitol fermentation, motility, pigment production, arabinose fermentation, and arginine hydrolysis tests were used for species-level identification (14-16).
Stool samples were screened for the presence of VRE by using bile esculin azide agar plus 6 µg/mL of vancomycin (BEAV, Himedia, India). After 24 h of incubation, if at least one colony had grown along with the darkening of the medium, then Gram stain and catalase tests were performed for presumptive identification of Enterococci (17). The microbiological tests used for species level identification were the same as described above in "Isolation and identification of Enterococcus spp. from clinical samples" (14-16).
Vancomycin susceptibility testing:
- Kirby-Bauer disc diffusion method was used to assess the susceptibility of enterococci isolated from clinical samples and stool samples to vancomycin. Discs containing 30 µg of vancomycin (Himedia, India) were used (18).
- Confirmation of vancomycin resistance and determination of vancomycin minimal inhibitory concentration (MIC) was performed using the macrobroth dilution method. Serial dilutions of vancomycin (Himedia, India) were prepared in brain-heart infusion (BHI) (Himedia, India) broth. The liquid culture of each bacterial isolate was standardized to 0.5 turbidity on the standard McFarland scale. An aliquot of 10 μL of each bacterial culture was inoculated in 500 μL of BHI broth with different concentrations of vancomycin ranging from 0.5 μg/mL to 512 μg/mL. Broths were incubated at 37 °C for 24 h. MIC was determined based on the vancomycin concentration inhibiting the bacterial isolate's visible growth evaluated in broth. Vancomycin MIC of ≥ 32 μg/mL was considered resistant, MIC of 8–16 μg/mL was considered intermediate resistance, and MIC of ≤ 4 μg/mL was considered sensitive (18,19).
Environmental swabs from VRE-infected and colonized patients’ surroundings, such as beds, trolleys, saline stands, side tables, and doorknobs, were collected to identify the transmission source in these patients (20). The samples were processed aerobically on bile esculin azide agar plus 6 µg/mL vancomycin (Himedia, India) (17). Any growth obtained was followed in the same manner as described above in “Isolation and identification of VRE from stool samples” (20,21).
Microsoft Excel spreadsheets were used for data entry and analysis. Statistical significance was calculated using the Chi-square test at 5% probability (p < 0.05).
Results
In the present study, 200 isolates of Enterococcus species were isolated from different clinical samples. Out of 200 isolates of Enterococcus species, 7 (3.5%) showed resistance to vancomycin by both disc diffusion and macrobroth dilution methods. Most VRE isolates (n = 5, 71.4%) were isolated (n = 5, 71.4%) from UTI patients.
Of 200 stool samples screened to assess VRE colonization, 12 patients (6%) had VRE. All Enterococcus isolated from patients infected (7/200) and colonized (12/200) with VRE were identified as E. faecium.
Of 7 patients with VRE infection, 5 (71.4%) were female, and 2 (28.6%) were males; female to male ratio was 2.5:1. Out of a total of 12 patients with VRE colonization, 9 patients (75.0%) were female and 3 (25.0%) were males. The female-to-male ratio was 3:1 in VRE-colonized patients. VRE infection was found to be more in the age group 61-70 years (n = 3, 42.9%), and VRE colonization was found to be more in the age group 41-60 years (n = 8, 66.7%) (Table 1).
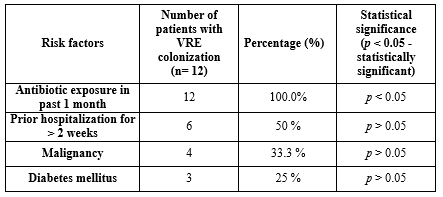
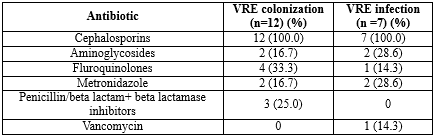
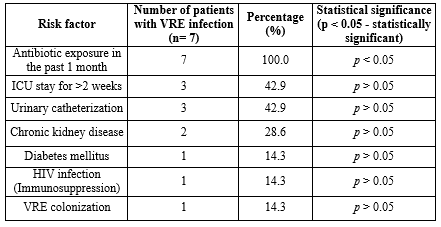
Risk factors associated with VRE infection and colonization: In the present study, all patients (100%) with VRE infection had a history of antibiotic exposure in the past month before VRE isolation, which showed a statistically significant association (p < 0.05). The most common antibiotics exposed were cephalosporins. Only one patient with VRE infection had a history of previous exposure to vancomycin (Table 2). Other underlying medical comorbid medical conditions did not show statistical significance with VRE infection, as demonstrated in Table 3.
In the present study, all patients (100.0%) with VRE colonization also had a history of antibiotic exposure, specifically cephalosporins, in the past month before VRE isolation, which was found to have a statistically significant association (p < 0.05). Other known risk factors like hospitalization for more than two weeks, malignancy, and diabetes were also analyzed but were not found to be significant. One colonized patient had an incidence of bloodstream infection caused by VRE (Table 4). All Environmental swabs collected from VRE-infected and colonized patients’ surroundings were negative for VRE.
Discussion
In the present study, urinary tract infection was the most common infection caused by VRE. Other authors have also shown a predominance of urinary tract infections caused by VRE (1,10,22). Enterococcus spp. are typically found in the genital and gastrointestinal tracts as normal commensal flora. They can enter the urinary tract in susceptible individuals with predisposing factors such as indwelling catheters, instrumentation, renal failure, kidney stones, or immunosuppression, leading to endogenous infection (23).
E. faecium was the only species isolated from all patients infected and colonized with VRE. Other studies conducted by Deshpande et al. (2013), Rahangdale et al. (2008), and Baragundi et al. (2010) also showed greater isolation of E. faecium among VRE isolates than any other Enterococcus species (24-26). E. faecium can acquire resistance against most of the available antibiotics by genetic transfer of resistance, which can be the reason for the higher isolation of this species from VRE isolates (27).
The female-to-male ratio of patients infected with VRE was 2.5:1. The predominance of VRE infection in females can be attributed to higher isolation of VRE in urinary tract infections, which is usually more common in females than males. Age groups commonly affected by VRE infection and VRE colonization were 41-70 years of age. The presence of comorbidities, such as diabetes mellitus, immunosuppression, and malignancies, are more common in the older age group, explaining more chances of infection and colonization with drug-resistant bacteria (10).
Various well-known risk factors and predisposing conditions associated with VRE infection are described in different studies, which include antibiotic exposure, prolonged hospitalization, invasive therapy, immunosuppression, diabetes mellitus, malignancy, and hepatorenal insufficiency (4,7-9,28). In the present study, prior antibiotic exposure was statistically associated with infection and colonization by VRE. Many other studies have shown similar findings (29-31).
Cephalosporins, fluoroquinolones, metronidazole, penicillins, and aminoglycosides were the other antibiotics to which VRE-infected and colonized patients were exposed in the present study. Previous vancomycin exposure in a patient causes selective pressure on the normal commensal flora and promotes the proliferation of VRE (6). In the present study, vancomycin exposure was present only in one patient (14.3%) of VRE infection. In previous studies, VRE infection and colonization were associated not only with vancomycin but mainly with cephalosporins, aminoglycosides, ciprofloxacin, and antibiotics used for anaerobes (10,28).
Other known risk factors, such as duration of ICU stay, invasive procedures, diabetes, immunosuppression, and malignancy, were not found to be statistically significant in the causation of infection and colonization by VRE. The reason could be the difference in sample size and prevalence of VRE (10).
One important finding in the present study was postoperative septicemia by VRE in a patient with gut colonization with VRE. This patient had undergone cardiovascular surgery and developed septicemia by VRE and died. If a patient is colonized with VRE, they have a 5-10-fold increased risk of developing serious endogenous infections with VRE (5). This highlights the need for screening of high-risk patients with predisposing factors for VRE colonization especially before any major surgical procedure, which prevents developing life-threatening infection. Also, if the infection develops in colonized patients, appropriate antibiotics should be started without delay. Necessary infection control measures can be taken while handling such colonized patients so that the spread of VRE to the environment and cross infections to other patients can be prevented (21).
Environmental swabs collected from the surroundings of VRE-infected and colonized patients were negative for VRE. This could be due to transient colonization of the surroundings by VRE (28). Other possible sources of VRE can be cross-infection from other VRE colonized patients, through the hands of healthcare workers, or through contaminated medical devices, which needs to be studied in detail (28,32,33).
Conclusion
Patients infected as well as colonized with VRE represent the actual burden of VRE. Screening high-risk patients is an important step in the prevention of the spread of VRE infection and its accurate treatment. Inappropriate use of antibiotics has led to the emergence of multidrug-resistant bacteria in healthcare settings. Antibiotic stewardship plays a major role in such a situation to reduce the inappropriate overuse of antibiotics, which can prevent the emergence of multidrug resistance in bacteria.
Acknowledgement
The authors wish to express their gratitude to Mrs. Varsha Pendse for her technical support.
Funding sources
None.
Ethical statement
The study was approved by the institutional ethics committee of B.J. Govt. Medical College, Pune (With protocol reference number BMC/IEC/Pharmac/D0313005-05) and was carried out following the approved guidelines.
Conflicts of interest
None.
Author contributions
All the authors have made substantial, direct, and intellectual contributions to the work.
Microsoft Excel spreadsheets were used for data entry and analysis. Statistical significance was calculated using the Chi-square test at 5% probability (p < 0.05).
Results
In the present study, 200 isolates of Enterococcus species were isolated from different clinical samples. Out of 200 isolates of Enterococcus species, 7 (3.5%) showed resistance to vancomycin by both disc diffusion and macrobroth dilution methods. Most VRE isolates (n = 5, 71.4%) were isolated (n = 5, 71.4%) from UTI patients.
Of 200 stool samples screened to assess VRE colonization, 12 patients (6%) had VRE. All Enterococcus isolated from patients infected (7/200) and colonized (12/200) with VRE were identified as E. faecium.
Of 7 patients with VRE infection, 5 (71.4%) were female, and 2 (28.6%) were males; female to male ratio was 2.5:1. Out of a total of 12 patients with VRE colonization, 9 patients (75.0%) were female and 3 (25.0%) were males. The female-to-male ratio was 3:1 in VRE-colonized patients. VRE infection was found to be more in the age group 61-70 years (n = 3, 42.9%), and VRE colonization was found to be more in the age group 41-60 years (n = 8, 66.7%) (Table 1).
|
Table 1. Age group-wise distribution of VRE infection and colonization
 |
|
Table 4. Risk factors associated with VRE colonization
 |
Discussion
In the present study, urinary tract infection was the most common infection caused by VRE. Other authors have also shown a predominance of urinary tract infections caused by VRE (1,10,22). Enterococcus spp. are typically found in the genital and gastrointestinal tracts as normal commensal flora. They can enter the urinary tract in susceptible individuals with predisposing factors such as indwelling catheters, instrumentation, renal failure, kidney stones, or immunosuppression, leading to endogenous infection (23).
E. faecium was the only species isolated from all patients infected and colonized with VRE. Other studies conducted by Deshpande et al. (2013), Rahangdale et al. (2008), and Baragundi et al. (2010) also showed greater isolation of E. faecium among VRE isolates than any other Enterococcus species (24-26). E. faecium can acquire resistance against most of the available antibiotics by genetic transfer of resistance, which can be the reason for the higher isolation of this species from VRE isolates (27).
The female-to-male ratio of patients infected with VRE was 2.5:1. The predominance of VRE infection in females can be attributed to higher isolation of VRE in urinary tract infections, which is usually more common in females than males. Age groups commonly affected by VRE infection and VRE colonization were 41-70 years of age. The presence of comorbidities, such as diabetes mellitus, immunosuppression, and malignancies, are more common in the older age group, explaining more chances of infection and colonization with drug-resistant bacteria (10).
Various well-known risk factors and predisposing conditions associated with VRE infection are described in different studies, which include antibiotic exposure, prolonged hospitalization, invasive therapy, immunosuppression, diabetes mellitus, malignancy, and hepatorenal insufficiency (4,7-9,28). In the present study, prior antibiotic exposure was statistically associated with infection and colonization by VRE. Many other studies have shown similar findings (29-31).
Cephalosporins, fluoroquinolones, metronidazole, penicillins, and aminoglycosides were the other antibiotics to which VRE-infected and colonized patients were exposed in the present study. Previous vancomycin exposure in a patient causes selective pressure on the normal commensal flora and promotes the proliferation of VRE (6). In the present study, vancomycin exposure was present only in one patient (14.3%) of VRE infection. In previous studies, VRE infection and colonization were associated not only with vancomycin but mainly with cephalosporins, aminoglycosides, ciprofloxacin, and antibiotics used for anaerobes (10,28).
Other known risk factors, such as duration of ICU stay, invasive procedures, diabetes, immunosuppression, and malignancy, were not found to be statistically significant in the causation of infection and colonization by VRE. The reason could be the difference in sample size and prevalence of VRE (10).
One important finding in the present study was postoperative septicemia by VRE in a patient with gut colonization with VRE. This patient had undergone cardiovascular surgery and developed septicemia by VRE and died. If a patient is colonized with VRE, they have a 5-10-fold increased risk of developing serious endogenous infections with VRE (5). This highlights the need for screening of high-risk patients with predisposing factors for VRE colonization especially before any major surgical procedure, which prevents developing life-threatening infection. Also, if the infection develops in colonized patients, appropriate antibiotics should be started without delay. Necessary infection control measures can be taken while handling such colonized patients so that the spread of VRE to the environment and cross infections to other patients can be prevented (21).
Environmental swabs collected from the surroundings of VRE-infected and colonized patients were negative for VRE. This could be due to transient colonization of the surroundings by VRE (28). Other possible sources of VRE can be cross-infection from other VRE colonized patients, through the hands of healthcare workers, or through contaminated medical devices, which needs to be studied in detail (28,32,33).
Conclusion
Patients infected as well as colonized with VRE represent the actual burden of VRE. Screening high-risk patients is an important step in the prevention of the spread of VRE infection and its accurate treatment. Inappropriate use of antibiotics has led to the emergence of multidrug-resistant bacteria in healthcare settings. Antibiotic stewardship plays a major role in such a situation to reduce the inappropriate overuse of antibiotics, which can prevent the emergence of multidrug resistance in bacteria.
Acknowledgement
The authors wish to express their gratitude to Mrs. Varsha Pendse for her technical support.
Funding sources
None.
Ethical statement
The study was approved by the institutional ethics committee of B.J. Govt. Medical College, Pune (With protocol reference number BMC/IEC/Pharmac/D0313005-05) and was carried out following the approved guidelines.
Conflicts of interest
None.
Author contributions
All the authors have made substantial, direct, and intellectual contributions to the work.
Research Article: Research Article |
Subject:
Microbiology
Received: 2023/06/15 | Accepted: 2024/05/20 | Published: 2024/11/12 | ePublished: 2024/11/12
Received: 2023/06/15 | Accepted: 2024/05/20 | Published: 2024/11/12 | ePublished: 2024/11/12
References
1. Ahmed MO, Baptiste KE. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb Drug Resist. 2018; 24(5): 590-606. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Karna A, Baral R, Khanal B. Characterization of Clinical Isolates of Enterococci with Special Reference to Glycopeptide Susceptibility at a Tertiary Care Center of Eastern Nepal. Int J Microbiol. 2019; 2019: 7936156. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Janjusevic A, Cirkovic I, Minic R, Stevanovic G, Soldatovic,I, Mihaljevic B, et al. Predictors of Vancomycin-Resistant Enterococcus spp. Intestinal Carriage among High-Risk Patients in University Hospitals in Serbia. Antibiotics. 2022; 11: 1228. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Hendrix CW, Hammond JM, Swoboda SM, Merz WG, Harrington SM, Perl TM, et al. Surveillance strategies and impact of vancomyc inresistant enterococcal colonization and infection in critically ill patients. Ann Surg .2001; 233 : 259-265. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Mainous MR, Lipsett PA, O'Brien M. Enterococcal bacteremia in the surgical intensive care unit. Does vancomycin resistance affect mortality? The Johns Hopkins SICU Study Group. Arch Surg. 1997; 132 : 76-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Kajihara T, Nakamura S, Iwanaga N, Oshima K , Takazono T , Miyazaki T, et al. Clinical characteristics and risk factors of enterococcal infections in Nagasaki, Japan: a retrospective study. BMC Infectious Diseases .2015; 15:426. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Montecalvo MA, Shay DK, Gedris C, Petrullo C, Uman J, Rodney K et al. A semiquantitative analysis of the fecal flora of patients with vancomycin-resistant Enterococci : colonized patients pose an infection control risk. Clin Infect Dis.1997; 25 : 929-930. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Correa-Martinez CL, Stollenwerk VB, Kossow A, Schaumburg F, Mellmann A, Kampmeier S. Risk Factors for Long-Term Vancomycin-Resistant Enterococci Persistence-A Prospective Longitudinal Study. Microorganisms. 2019; 7(10): 400. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Se YB, Chun HJ, Yi HJ, Dong-Won Kim DW, Yong Ko Y, Oh SJ. Incidence and Risk Factors of Infection Caused by Vancomycin-Resistant Enterococcus Colonization in Neurosurgical Intensive Care Unit Patients. J Korean Neurosurg Soc. 2009; 46: 123-129. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Bhatt P, Patel A, Sahni A, Grover N, Chaudhari C , Nikunja Kumar Das, et al. Emergence of multidrug resistant Enterococci at a tertiary care centre. Med J Armed Forces India. 2015; 71(2): 139-144. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Dadfarma N, Imani FA, Oskoui M, Hosseini H. "High level of gentamicin resistance (HLGR) among Enterococcus strains isolated from clinical specimens. J Infect Public Health. 2013; 6(3): 202-208. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Jia W, Li G, Wang W. Prevalence and antimicrobial resistance of Enterococcus species: a hospital-based study in China. Int J Environ Res Public Health. 2014; 11(3): 3424-3442. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Biswas P, Dey S, Adhikari L, Sen A. Detection of vancomycin resistance in Enterococcus species isolated from clinical samples and feces of colonized patients by phenotypic and genotypic methods. Indian J Pathol Microbiol. 2016; 59(2): 188-93. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Mikulska M, Del BV, Prinapori R, Boni L, Raiola AM, Gualandi F, et al. Risk factors for Enterococcal bacteremia in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2010; 12(6): 505-12. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Weinstock DM, Conlon M, Iovino C, Aubrey T, Gudiol C, Riedel E, et al. Colonization, bloodstream infection, and mortality caused by vancomycin resistant Enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007; 13(5): 615-21. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Vydra J, Shanley RM, George I, Ustun C, Smith AR, Weisdorf DJ, et al. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012; 55(6): 764-70. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Buetti N, Wassilew N, Rion V, Senn L, Gardiol C, Widmer A, et al. Emergence of vancomycin-resistant Enterococci in Switzerland: a nation-wide survey. Antimicrobial Resistance and Infection Control. 2019; 8:16. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Uddhav SB, Sivagurunathan MS. Antibiotic Susceptibility Testing: A Review On Current Practices. Int J Pharm 2016; 6(3): 11-17. [View at Publisher]
19. CLSI. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-third Informational Supplement, M100-S23. Vol. 33. Wayne, USA;, CLSI; 2008. 90-3. [View at Publisher] [Google Scholar]
20. Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant Enterococci. Clin Microbiol Rev 2000; 13(4): 686-707. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Betty AF, Daniel LS, Weissfeld AS, editors. Overview of bacterial identifications methods and strategies. In: Bailey & Scott's Diagnostic Microbiology. 12th ed. Missouri: Mosby Elsevier; 2007: 216-41. [View at Publisher] [Google Scholar]
22. Jenkins SG, Raskoshina L, Schuetz AN. Comparison of Performance of the Novel Chromogenic Spectra VRE Agar to That of Bile Esculin Azide and Campylobacter Agars for Detection of Vancomycin-Resistant Enterococci in Fecal Samples. J Clin Microbiol. 2011; 49(11): 3947-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC, editors. Gram-positive cocci Part 2: Streptococci, Enterococci and the Streptococcus like bacteria. In: Colour Atlas and Text Book of Diagnostic Microbiology. 6th ed. Philadelphia: Lippincott; 2006. p. 725-33.
24. Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Special Phenotypic Methods for Detecting Antibacterial Resistance. In: Manual of Clinical Microbiology. 9th ed. Washington, DC: ASM Press; 2007. p. 1152-72. [View at Publisher] [Google Scholar]
25. Center for disease control and prevention. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Morb Mortal Wkly Rep 1995; 44(RR- 12):1-13. 1995;44(RR-12):1-13. [View at Publisher] [Google Scholar]
26. Center for disease control and prevention. Vancomycin resistant Enterococci in health care settings. HAI. 2024. [View at Publisher]
27. Pan S, Wang J, Chen Y, Chang Y, Chen M, Chang C. Incidence of and Risk Factors for Infection or Colonization of Vancomycin-Resistant Enterococci in Patients in the Intensive Care Unit Acute renal failure. PLoS One. 2012;7(10):e47297. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Heintz BH, Halilovic J, Christensen CL. Vancomycin resistant Enterococcal Urinary Tract Infections. Pharmacotherapy. 2010; 30(11): 1136-49. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Deshpande VR, Karmarkar MG, Mehta PR. Letter to the Editor Prevalence of multidrug-resistant Enterococci in a tertiary care hospital in. J Infect Dev Ctries. 2013; 7(2): 155-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Rahangdale V, Agrawal G. Study of antimicrobial resistance in Enterococci. Indian J Med Microbiol. 2008; 26(3): 285-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Baragundi M, Sonth S, Solabannavar S, Patil C. Species Prevalence And Antimicrobial Resistance Pattern Of Enterococcal Isolates In A Tertiary Health Care Centre. J Clin Diagnostic Res. 2010; 4: 3405-9. [View at Publisher] [DOI]
32. Tacconelli E, Cataldo MA. Vancomycin-resistant Enterococci (VRE) : transmission and control. Int J Antimicrob Agents. 2008; 31(2): 99-106. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Taneja N, Rani P, Emmanuel R, Sharma M. Significance of vancomycin resistant Enterococci from urinary specimens at a tertiary care centre in Northern India. Indian J Med Res. 2004; 119: 72-4. [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com