Volume 19, Issue 1 (Jan-Feb 2025)
mljgoums 2025, 19(1): 12-18 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Okikiade A, Kanu C, Iyapo O, Omitogun O. Investigating the roles of cytokines and chemokines in pregnancy-related toxemia. mljgoums 2025; 19 (1) :12-18
URL: http://mlj.goums.ac.ir/article-1-1813-en.html
URL: http://mlj.goums.ac.ir/article-1-1813-en.html
1- Department of Pathology, Clinical Sciences, California Northstate University, Elk Grove, CA, USA ; Ejyde International Education and Research Consultancy, LCC, USA , okikis@yahoo.com
2- Ejyde International Education and Research Consultancy, LCC, USA
3- Department of Pathology, Eko University of Medicine and Health Sciences, Lagos, Nigeria
4- Alluring Healthcare Solutions, LLC, USA
2- Ejyde International Education and Research Consultancy, LCC, USA
3- Department of Pathology, Eko University of Medicine and Health Sciences, Lagos, Nigeria
4- Alluring Healthcare Solutions, LLC, USA
Full-Text [PDF 722 kb]
(708 Downloads)
| Abstract (HTML) (2994 Views)
Cytokines and chemokines share similar structures and functions, and are clustered into groups of interdependent homologs. They exhibit functional redundancy and have a widespread impact on other groups of cytokines or chemokines.
IL-1/IL-18/IL-12
The IL-1-related group of pro-inflammatory cytokines consists of IL-1α, IL-1β, IL-1 receptor antagonist (IL-1RA), and IL-18 (12,13). IL-1α and IL-1β are produced mainly by mononuclear and epithelial cells upon inflammation, injury, and infection (2,14). IL-18 shares biological function with IL-12 in that it induces IFN-γ secretion (In synergy with IL-12), enhances natural killer (NK) cell activity, and promotes inflammatory Th1 cell responses (15). IL-2 is commonly regarded as an autocrine or paracrine T cell growth factor, but it affects many cell types, such as B cells, NK cells, macrophages, and neutrophils. IL-12 is essential for cell-mediated immunity, acting as a crucial cytokine that shifts the balance between Th1 and Th2 cells towards a Th1 predominance.
IL-10, IL-6, TNF, and related family
IL-10, IL-19, and IL-20 are members of a related group of interleukins, homologous to IL-10. IL-10 plays a crucial role in suppressing inflammatory responses. This is accomplished through inhibiting the synthesis of IFN-γ, IL-2, IL-3, TNF-α, and GM-CSF by cells such as macrophages and Th1 cells (14,15,16). New members of the TNF family have been recently explored, such as TNF-α, TNF-β, and lymphotoxin (LT)-β (17). The transforming growth factor (TGF)-β family consists of more than 30 members and is involved in the development, immune regulation, immune tolerance, carcinogenesis, tissue repair, and the generation and differentiation of cells (2).
The consequent decrease in uteroplacental blood flow leads to decreased oxygen delivery to the placenta and impaired placental function (18). This causes the placenta to express antiangiogenic factors and pro-inflammatory cytokines, thereby playing a role in developing toxemia during pregnancy (2). IL-6 and TNFα are the primary and most abundant pro-inflammatory cytokines regulating the maternal immune system (2). These cytokines regulate the function of endothelial cells by making the vessels more permeable and inducing apoptosis of the trophoblastic cells. Women with toxemia of pregnancy produce significantly higher levels of pro-inflammatory cytokines compared to average pregnant women. In contrast, average pregnant women show substantially greater Th2 cytokines IL-4 and IL-5 production than those in normal pregnancies.
An elevation in the levels of IL-6 and TNF-α in pre-eclamptic placental tissues has been documented. ELISA analysis of the maternal serum of pre-eclamptic subjects has demonstrated a significant upregulation of cytokines. The concentrations of pro-inflammatory cytokines steadily increase from the 28th week of gestation until term in
both the placenta and serum of mothers with pre-eclampsia. Assessment of intracellular cytokines using flow cytometry has spotlighted a shift towards Th1-type in Toxemia of pregnancy. Numerous studies have explored cytokine production by peripheral blood mononuclear cells (PBMC). Maternal PBMCs generate elevated levels of pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-2, IL-1, IL-6, and IL-8.
Lockwood et al. presented evidence of increased IL-6 mRNA and protein in leukocyte-free decidual cells from individuals with PE. Recent research has revealed the capacity of human endometrial endothelial cells to phagocytose apoptotic trophoblasts and subsequently release the pro-inflammatory cytokine IL-6. This mechanism can justify the observed inflammatory response in pre-eclamptic placentas (19).
IL-17 and T lymphocytes
IL-17 is a potent pro-inflammatory cytokine with a significant role in the pathogenesis of autoimmune diseases (20). The lymphocytic cells exhibiting antagonistic functions include T-regulatory cells (Tregs) and T-helper 17 cells (Th17). Tregs are essential elements in pregnancy that play a vital role in preventing the mother’s immune system from attacking the fetal tissue (21,22). The decreased amount of Treg is due to improper implantation. Th17 cells contribute to inflammation, autoimmunity, and transplant rejection in humans. Many obstetric complications have been associated with a substantial increase in Th17 cells and a decrease in Tregs. Maintaining a balance and correlation between Th1 cells, Th2 cells, Th17 cells, and Tregs is imperative for creating a secure environment for the fetus and ensuring safe delivery (23). Interleukin-17, an inflammatory cytokine, is secreted by Th17 cells. It plays a significant role in the progression of numerous inflammatory processes. It is found in CD4+ cells, CD8+ cells, NK cells, and monocytes; human IL-17 functions dynamically in the recruitment and activation processes.
Tumor necrosis factor-alpha and toxemia of pregnancy
Tumor necrosis factor-alpha (TNF-alpha) is a pro-inflammatory cytokine synthesized by macrophages (14,19,21), T-lymphocytes, natural killer cells, and monocytes (16,21,24). It is released during the acute phase of inflammation and orchestrates various signals to facilitate necrosis or apoptosis. This protein is also crucial for strengthening the immune system against infections. Several studies have shown an increase in these cytokines in toxemia of pregnancy. The generation and release of TNF-α are influenced by hypoxia-reoxygenation resulting from the intermittent perfusion of the placenta (16,25).
TNF-α binds to two distinct receptors, enabling signal transduction through the pathway and stimulating diverse cellular responses, which regulate cell survival, differentiation, inflammation, cell defense, and cell proliferation (Figure 4). Excessive and sustained activation of TNF-α could lead to chronic inflammation, autoimmune diseases, and other complications (26). Knowledge of the TNF-α signaling pathway has expanded and led to the advent of therapeutic approaches for the treatment of immunologic diseases, notably TNF-α inhibitors.
TNF-α is a cytokine that works on different types of cells to regulate inflammatory responses (24). It also plays a vital role in the pathogenesis of specific inflammation, cancers, and autoimmune diseases. Functionally, TNF-α initiates a cascade of inflammatory molecules, including other cytokines and chemokines. It exists in both a soluble and transmembrane form, with transmembrane TNF-α (tmTNF-α) representing the initially synthesized precursor that requires processing by TNF-α-converting enzyme (TACE), a membrane-bound metalloproteinase, to be released as soluble TNF-α (sTNF-α). The soluble TNF-α is released and binds to the receptors, initiating a cascade of reactions leading to the release of molecules that stimulate apoptosis, inflammation, and cell survival (27).
These processes lead to placental hypoxia and, consequently, ischemia. This reduced oxygen within the placental tissue can produce and release cytotoxic factors, which may affect the maternal blood supply during gestation. Inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), known to induce dysfunction in endothelial cells, are synthesized and released in the human placenta of patients with toxemia of pregnancy.
Recent reports indicate that hypoxia increases the production of TNF-α and IL-1 from the human placenta. Placental cells also express erythropoietin (EPO), a molecule regulated transcriptionally by hypoxia in mammals (25,28). Intriguingly, TNF-α and IL-1 exhibit DNA sequences homologous to the hypoxia-responsive enhancer element of the EPO gene, suggesting a potential, albeit untested, molecular link between placental hypoxia and the stimulation of cytokine production. Inflammatory cytokines excessively produced by the placenta in response to hypoxia may lead to their elevated plasma levels and induce endothelial activation and dysfunction in preeclampsia.
Full-Text: (572 Views)
Introduction
Toxemia of pregnancy is the spectrum of hypertensive disorders in pregnancy, ranging from mild hypertension and preeclampsia to eclampsia. Toxemia during pregnancy can be complicated by intrauterine growth restriction, placental abruption, stillbirth, HELLP syndrome (Hemolysis, elevated liver enzymes, and low platelets), and DIC (Disseminated intravascular coagulation). Preeclampsia (PE) manifests as high blood pressure and weight gain with protein in the urine after 20 weeks of gestation (1), and the co-occurrence of neurological manifestations is termed eclampsia or imminent eclampsia. Other manifestations are intense headaches, visual disturbances such as blurring or flashing, discomfort below the ribs, nausea, and abrupt swelling of the face, hands, and feet.
The exact cause of toxemia in pregnancy remains unclear, but it is believed to be related to inflammation, which is a key factor in preeclampsia. A disproportion in lymphocytic (TH1, TH2, and TH17) immune responses and endothelial dysfunction is apparent. Likewise, cytokines such as interleukins and TNF-alpha play crucial roles (2). A dysfunctional placenta and the disruption of the interaction between the fetal blood supply and the maternal circulation are implicated.
Immune-mediated inflammation triggers placental hypoperfusion, resulting in low birth weight and intrauterine growth retardation. Oxidative stress is associated with activation of the maternal inflammatory response, such as regulatory T cells, B cells, macrophages, natural killer cells, and neutrophils, which are known to have major causative roles in the pathology of preeclampsia. The roles of inflammatory cytokines and autoantibodies against the angiotensin II type 1 receptor are now recognized.
According to the American College of Obstetricians and Gynecologists guidelines, a hypertensive emergency during pregnancy is characterized by the sudden onset of severe hypertension, with systolic blood pressure exceeding 160 mmHg or diastolic blood pressure surpassing 110 mmHg, especially in the context of preeclampsia or eclampsia (3,4). This typically presents as the emergence of new-onset hypertension and proteinuria in the third trimester. Preeclampsia can advance quickly, leading to severe complications such as reduced uterine perfusion, placental abruption, premature delivery, and increased risks of death for both the mother and the fetus without appropriate management (5,6).
Conceptualized pathophysiology
The pathology of toxemia of pregnancy is unclear. Genetic, microbial, and immunological mechanisms play a role in the pathogenesis of toxemia of pregnancy (Figure 1). The first step is abnormal placentation, leading to placental ischemia. Oxidative stress develops and leads to increased release of pro-inflammatory cytokines, increased antiangiogenetic factors, and decreased placental growth factors, followed by endothelial dysfunction (7). Subsequently, dendritic cells responsible for implantation support and macrophages that support placentation are destroyed, and vascular remodeling occurs, which triggers the production of danger signals such as damage-associated molecular patterns (DAMPs) (8). DAMPs are nuclear or cytosolic proteins with defined intracellular functions that promote an inflammatory response by binding to pattern recognition receptors. RNA, DNA, IL-1 alpha, reactive oxygen species (ROS), ATP, and fibronectin are examples of DAMPs released in the placenta and endothelial injury (8,9).
Toxemia of pregnancy is the spectrum of hypertensive disorders in pregnancy, ranging from mild hypertension and preeclampsia to eclampsia. Toxemia during pregnancy can be complicated by intrauterine growth restriction, placental abruption, stillbirth, HELLP syndrome (Hemolysis, elevated liver enzymes, and low platelets), and DIC (Disseminated intravascular coagulation). Preeclampsia (PE) manifests as high blood pressure and weight gain with protein in the urine after 20 weeks of gestation (1), and the co-occurrence of neurological manifestations is termed eclampsia or imminent eclampsia. Other manifestations are intense headaches, visual disturbances such as blurring or flashing, discomfort below the ribs, nausea, and abrupt swelling of the face, hands, and feet.
The exact cause of toxemia in pregnancy remains unclear, but it is believed to be related to inflammation, which is a key factor in preeclampsia. A disproportion in lymphocytic (TH1, TH2, and TH17) immune responses and endothelial dysfunction is apparent. Likewise, cytokines such as interleukins and TNF-alpha play crucial roles (2). A dysfunctional placenta and the disruption of the interaction between the fetal blood supply and the maternal circulation are implicated.
Immune-mediated inflammation triggers placental hypoperfusion, resulting in low birth weight and intrauterine growth retardation. Oxidative stress is associated with activation of the maternal inflammatory response, such as regulatory T cells, B cells, macrophages, natural killer cells, and neutrophils, which are known to have major causative roles in the pathology of preeclampsia. The roles of inflammatory cytokines and autoantibodies against the angiotensin II type 1 receptor are now recognized.
According to the American College of Obstetricians and Gynecologists guidelines, a hypertensive emergency during pregnancy is characterized by the sudden onset of severe hypertension, with systolic blood pressure exceeding 160 mmHg or diastolic blood pressure surpassing 110 mmHg, especially in the context of preeclampsia or eclampsia (3,4). This typically presents as the emergence of new-onset hypertension and proteinuria in the third trimester. Preeclampsia can advance quickly, leading to severe complications such as reduced uterine perfusion, placental abruption, premature delivery, and increased risks of death for both the mother and the fetus without appropriate management (5,6).
Conceptualized pathophysiology
The pathology of toxemia of pregnancy is unclear. Genetic, microbial, and immunological mechanisms play a role in the pathogenesis of toxemia of pregnancy (Figure 1). The first step is abnormal placentation, leading to placental ischemia. Oxidative stress develops and leads to increased release of pro-inflammatory cytokines, increased antiangiogenetic factors, and decreased placental growth factors, followed by endothelial dysfunction (7). Subsequently, dendritic cells responsible for implantation support and macrophages that support placentation are destroyed, and vascular remodeling occurs, which triggers the production of danger signals such as damage-associated molecular patterns (DAMPs) (8). DAMPs are nuclear or cytosolic proteins with defined intracellular functions that promote an inflammatory response by binding to pattern recognition receptors. RNA, DNA, IL-1 alpha, reactive oxygen species (ROS), ATP, and fibronectin are examples of DAMPs released in the placenta and endothelial injury (8,9).
.PNG) Figure 1. Schematic summary of the pathogenesis of toxemia of pregnancy |
The importance of ncRNAs as clinical biomarkers has been explored in many human diseases, including pregnancy-related hypertension. There is evidence of the involvement of placenta-expressed miRNAs and lncRNAs in the immunological regulation of crucial processes of placenta development and function during pregnancy. Abnormal expression of these molecules is related to immune pathophysiological processes that occur during preeclampsia. Multiple ncRNAs are involved in the PE immune dysregulation, participating in type 1 immune response regulation, immune microenvironment regulation in the placenta, promoting inflammatory factors, trophoblast cell invasion in women with early-onset PE (EOPE), placental development and angiogenesis, and autophagy (8,9).
Roles of endothelin in the pathogenesis of preeclampsia
A group of researchers introduced a 2-stage model of preeclampsia. In Stage 1, placental perfusion decreases, leading to hypoxic injury in the fetus, and further proposed that partial persistence of hypoxia initiates a chain of events leading to toxemia during pregnancy in three sequential stages (Figure 2). The first stage results in the retention of the "endothelin-producing" endothelium in uteroplacental arteries secondary to the incomplete physiological transformation of the vessels (7-9). Consequently, the uteroplacental vessels become reactive to pathologic signals, which drives local arteriopathy. In the second stage, uteroplacental blood flow progressively decreases, leading to oxidative stress in the placenta (Figure 2).
Roles of endothelin in the pathogenesis of preeclampsia
A group of researchers introduced a 2-stage model of preeclampsia. In Stage 1, placental perfusion decreases, leading to hypoxic injury in the fetus, and further proposed that partial persistence of hypoxia initiates a chain of events leading to toxemia during pregnancy in three sequential stages (Figure 2). The first stage results in the retention of the "endothelin-producing" endothelium in uteroplacental arteries secondary to the incomplete physiological transformation of the vessels (7-9). Consequently, the uteroplacental vessels become reactive to pathologic signals, which drives local arteriopathy. In the second stage, uteroplacental blood flow progressively decreases, leading to oxidative stress in the placenta (Figure 2).
.PNG) Figure 2. Toxemia of pregnancy stages |
ETs are a family of pro-inflammatory cytokines consisting of several amino acids, the major ones being ET-1, ET-2, and ET-3, encoded by different genes (Endothelin1, 2, and 3). These genes code for the pre-pro form of ETs (Pre-pro), the precursors cleaved by cellular endopeptidases into the inactive big ETs. Additional alteration by one of the ET-converting enzymes (ECEs) releases biologically active endothelin products (7-9).
ET-1 is the most abundant member of the family and is secreted by the syncytiotrophoblast and endothelium on the basolateral side (7-9). Upon stimulation, it is secreted from the Weibel-Palade bodies of the endothelial cells. Several enzymes, hormones, and cytokines, such as angiotensin II, hypoxia, growth factors, and epinephrine, have been shown to increase the stimulation of ET-1 release (7).
The ETA binds mostly to ET-1 and ET-2 rather than other endothelin receptors, inducing vasoconstriction of placental and maternal blood vessels. Studies on ET-1 in normal and preeclamptic pregnancies have shown a triple increase in endothelin-1 in women with toxemia of pregnancy as compared to normal pregnancy. The primary reason for this is not fully understood (7).
The release of endothelin-1 triggers oxidative stress in the placenta, leading to increased production of factors such as soluble FMS-like tyrosine kinase-1 (sFLT-1). There is evidence that endothelial dysfunction deteriorates with sFLT-1 secretion into the circulation, where it antagonizes the activity of vascular endothelial growth factor and placental growth factor (2,7-9).
Microbial interplay
Toll-like receptor signaling (TLRs) activates nuclear factor-κB (NF-κB) dependent and independent pathways to produce cytokines and chemokines. Trophoblast TLR-3 and TLR-4 activation by microbial byproducts and chemokine secretion initiates the innate immune response, and the decidua becomes infiltrated with pNK cells and macrophages, which microbes can induce. Double-stranded RNA (dsRNA) and single-stranded RNA (ssRNA) upregulate the expression of TLR3, TLR7, and TLR8 in mouse placentae, leading to pregnancy-associated hypertension, endothelial dysfunction, and placental inflammation.
The role of γδ T cells has not yet been determined in preeclampsia. However, an increase in the production of pro-inflammatory stimuli, interferon (IFN)-γ and IL-17 by γδ T cells has been reported in women with idiopathic recurrent pregnancy loss (10).
Cytokines
Cytokines facilitate cell interactions and communication. They can be grouped into chemokines (Cytokines with chemotactic activities) and interleukins (Cytokines produced by leukocytes). Their functions can be seen in diverse cells of the kidneys, brain, liver, heart, and blood. These proteins mediate inflammatory responses and promote the synthesis of other interleukins. Imbalance of these cytokines can cause several complications, such as disruption of the vascular system, leading to toxemia during pregnancy. The ratios of Th2 to Th1 cytokines indicate that Th1-proinflammatory cytokine production is increased in preeclampsia (10). Flow cytometry investigations have demonstrated a shift toward Th1-type reactivity in preeclampsia.
These interacting biological signals have remarkable functions, such as influencing growth and development, hematopoiesis, lymphocyte recruitment, T cell subset differentiation, and inflammation. Mature CD4 and CD8 T cells leave the thymus with a naive phenotype and produce a variety of cytokines. In the periphery, these T cells encounter antigen-presenting cells (APCs) displaying either major histocompatibility complex (MHC) class I molecules (peptides generated in the CD8 T cell cytosols) or MHC class II molecules (Peptides degraded in CD4 T cells' intracellular vesicles). Following activation, characteristic cytokine and chemokine secretion profiles allow the classification of CD4 T helper (Th) cells into two significant subpopulations in mice and humans. Th1 cells secrete IL-2, interferon-γ (IFN-γ), and tumor necrosis factor-β (TNF-β), whereas Th2 cells secrete IL-4, IL-5, IL-6, IL-10, and IL-13 (11) (Figure 3). Th1 cells are integral to cell-mediated immunity and promote inflammation, cytotoxicity, and delayed-type hypersensitivity (DTH). Th2 cells are pillars of humoral immunity and downregulate the inflammatory actions of Th1 cells.
ET-1 is the most abundant member of the family and is secreted by the syncytiotrophoblast and endothelium on the basolateral side (7-9). Upon stimulation, it is secreted from the Weibel-Palade bodies of the endothelial cells. Several enzymes, hormones, and cytokines, such as angiotensin II, hypoxia, growth factors, and epinephrine, have been shown to increase the stimulation of ET-1 release (7).
The ETA binds mostly to ET-1 and ET-2 rather than other endothelin receptors, inducing vasoconstriction of placental and maternal blood vessels. Studies on ET-1 in normal and preeclamptic pregnancies have shown a triple increase in endothelin-1 in women with toxemia of pregnancy as compared to normal pregnancy. The primary reason for this is not fully understood (7).
The release of endothelin-1 triggers oxidative stress in the placenta, leading to increased production of factors such as soluble FMS-like tyrosine kinase-1 (sFLT-1). There is evidence that endothelial dysfunction deteriorates with sFLT-1 secretion into the circulation, where it antagonizes the activity of vascular endothelial growth factor and placental growth factor (2,7-9).
Microbial interplay
Toll-like receptor signaling (TLRs) activates nuclear factor-κB (NF-κB) dependent and independent pathways to produce cytokines and chemokines. Trophoblast TLR-3 and TLR-4 activation by microbial byproducts and chemokine secretion initiates the innate immune response, and the decidua becomes infiltrated with pNK cells and macrophages, which microbes can induce. Double-stranded RNA (dsRNA) and single-stranded RNA (ssRNA) upregulate the expression of TLR3, TLR7, and TLR8 in mouse placentae, leading to pregnancy-associated hypertension, endothelial dysfunction, and placental inflammation.
The role of γδ T cells has not yet been determined in preeclampsia. However, an increase in the production of pro-inflammatory stimuli, interferon (IFN)-γ and IL-17 by γδ T cells has been reported in women with idiopathic recurrent pregnancy loss (10).
Cytokines
Cytokines facilitate cell interactions and communication. They can be grouped into chemokines (Cytokines with chemotactic activities) and interleukins (Cytokines produced by leukocytes). Their functions can be seen in diverse cells of the kidneys, brain, liver, heart, and blood. These proteins mediate inflammatory responses and promote the synthesis of other interleukins. Imbalance of these cytokines can cause several complications, such as disruption of the vascular system, leading to toxemia during pregnancy. The ratios of Th2 to Th1 cytokines indicate that Th1-proinflammatory cytokine production is increased in preeclampsia (10). Flow cytometry investigations have demonstrated a shift toward Th1-type reactivity in preeclampsia.
These interacting biological signals have remarkable functions, such as influencing growth and development, hematopoiesis, lymphocyte recruitment, T cell subset differentiation, and inflammation. Mature CD4 and CD8 T cells leave the thymus with a naive phenotype and produce a variety of cytokines. In the periphery, these T cells encounter antigen-presenting cells (APCs) displaying either major histocompatibility complex (MHC) class I molecules (peptides generated in the CD8 T cell cytosols) or MHC class II molecules (Peptides degraded in CD4 T cells' intracellular vesicles). Following activation, characteristic cytokine and chemokine secretion profiles allow the classification of CD4 T helper (Th) cells into two significant subpopulations in mice and humans. Th1 cells secrete IL-2, interferon-γ (IFN-γ), and tumor necrosis factor-β (TNF-β), whereas Th2 cells secrete IL-4, IL-5, IL-6, IL-10, and IL-13 (11) (Figure 3). Th1 cells are integral to cell-mediated immunity and promote inflammation, cytotoxicity, and delayed-type hypersensitivity (DTH). Th2 cells are pillars of humoral immunity and downregulate the inflammatory actions of Th1 cells.
.PNG) Figure 3. Cytokine production by T cells (+ Stimulatory, - Inhibitory) |
Cytokines and chemokines share similar structures and functions, and are clustered into groups of interdependent homologs. They exhibit functional redundancy and have a widespread impact on other groups of cytokines or chemokines.
IL-1/IL-18/IL-12
The IL-1-related group of pro-inflammatory cytokines consists of IL-1α, IL-1β, IL-1 receptor antagonist (IL-1RA), and IL-18 (12,13). IL-1α and IL-1β are produced mainly by mononuclear and epithelial cells upon inflammation, injury, and infection (2,14). IL-18 shares biological function with IL-12 in that it induces IFN-γ secretion (In synergy with IL-12), enhances natural killer (NK) cell activity, and promotes inflammatory Th1 cell responses (15). IL-2 is commonly regarded as an autocrine or paracrine T cell growth factor, but it affects many cell types, such as B cells, NK cells, macrophages, and neutrophils. IL-12 is essential for cell-mediated immunity, acting as a crucial cytokine that shifts the balance between Th1 and Th2 cells towards a Th1 predominance.
IL-10, IL-6, TNF, and related family
IL-10, IL-19, and IL-20 are members of a related group of interleukins, homologous to IL-10. IL-10 plays a crucial role in suppressing inflammatory responses. This is accomplished through inhibiting the synthesis of IFN-γ, IL-2, IL-3, TNF-α, and GM-CSF by cells such as macrophages and Th1 cells (14,15,16). New members of the TNF family have been recently explored, such as TNF-α, TNF-β, and lymphotoxin (LT)-β (17). The transforming growth factor (TGF)-β family consists of more than 30 members and is involved in the development, immune regulation, immune tolerance, carcinogenesis, tissue repair, and the generation and differentiation of cells (2).
The consequent decrease in uteroplacental blood flow leads to decreased oxygen delivery to the placenta and impaired placental function (18). This causes the placenta to express antiangiogenic factors and pro-inflammatory cytokines, thereby playing a role in developing toxemia during pregnancy (2). IL-6 and TNFα are the primary and most abundant pro-inflammatory cytokines regulating the maternal immune system (2). These cytokines regulate the function of endothelial cells by making the vessels more permeable and inducing apoptosis of the trophoblastic cells. Women with toxemia of pregnancy produce significantly higher levels of pro-inflammatory cytokines compared to average pregnant women. In contrast, average pregnant women show substantially greater Th2 cytokines IL-4 and IL-5 production than those in normal pregnancies.
An elevation in the levels of IL-6 and TNF-α in pre-eclamptic placental tissues has been documented. ELISA analysis of the maternal serum of pre-eclamptic subjects has demonstrated a significant upregulation of cytokines. The concentrations of pro-inflammatory cytokines steadily increase from the 28th week of gestation until term in
both the placenta and serum of mothers with pre-eclampsia. Assessment of intracellular cytokines using flow cytometry has spotlighted a shift towards Th1-type in Toxemia of pregnancy. Numerous studies have explored cytokine production by peripheral blood mononuclear cells (PBMC). Maternal PBMCs generate elevated levels of pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-2, IL-1, IL-6, and IL-8.
Lockwood et al. presented evidence of increased IL-6 mRNA and protein in leukocyte-free decidual cells from individuals with PE. Recent research has revealed the capacity of human endometrial endothelial cells to phagocytose apoptotic trophoblasts and subsequently release the pro-inflammatory cytokine IL-6. This mechanism can justify the observed inflammatory response in pre-eclamptic placentas (19).
IL-17 and T lymphocytes
IL-17 is a potent pro-inflammatory cytokine with a significant role in the pathogenesis of autoimmune diseases (20). The lymphocytic cells exhibiting antagonistic functions include T-regulatory cells (Tregs) and T-helper 17 cells (Th17). Tregs are essential elements in pregnancy that play a vital role in preventing the mother’s immune system from attacking the fetal tissue (21,22). The decreased amount of Treg is due to improper implantation. Th17 cells contribute to inflammation, autoimmunity, and transplant rejection in humans. Many obstetric complications have been associated with a substantial increase in Th17 cells and a decrease in Tregs. Maintaining a balance and correlation between Th1 cells, Th2 cells, Th17 cells, and Tregs is imperative for creating a secure environment for the fetus and ensuring safe delivery (23). Interleukin-17, an inflammatory cytokine, is secreted by Th17 cells. It plays a significant role in the progression of numerous inflammatory processes. It is found in CD4+ cells, CD8+ cells, NK cells, and monocytes; human IL-17 functions dynamically in the recruitment and activation processes.
Tumor necrosis factor-alpha and toxemia of pregnancy
Tumor necrosis factor-alpha (TNF-alpha) is a pro-inflammatory cytokine synthesized by macrophages (14,19,21), T-lymphocytes, natural killer cells, and monocytes (16,21,24). It is released during the acute phase of inflammation and orchestrates various signals to facilitate necrosis or apoptosis. This protein is also crucial for strengthening the immune system against infections. Several studies have shown an increase in these cytokines in toxemia of pregnancy. The generation and release of TNF-α are influenced by hypoxia-reoxygenation resulting from the intermittent perfusion of the placenta (16,25).
TNF-α binds to two distinct receptors, enabling signal transduction through the pathway and stimulating diverse cellular responses, which regulate cell survival, differentiation, inflammation, cell defense, and cell proliferation (Figure 4). Excessive and sustained activation of TNF-α could lead to chronic inflammation, autoimmune diseases, and other complications (26). Knowledge of the TNF-α signaling pathway has expanded and led to the advent of therapeutic approaches for the treatment of immunologic diseases, notably TNF-α inhibitors.
TNF-α is a cytokine that works on different types of cells to regulate inflammatory responses (24). It also plays a vital role in the pathogenesis of specific inflammation, cancers, and autoimmune diseases. Functionally, TNF-α initiates a cascade of inflammatory molecules, including other cytokines and chemokines. It exists in both a soluble and transmembrane form, with transmembrane TNF-α (tmTNF-α) representing the initially synthesized precursor that requires processing by TNF-α-converting enzyme (TACE), a membrane-bound metalloproteinase, to be released as soluble TNF-α (sTNF-α). The soluble TNF-α is released and binds to the receptors, initiating a cascade of reactions leading to the release of molecules that stimulate apoptosis, inflammation, and cell survival (27).
These processes lead to placental hypoxia and, consequently, ischemia. This reduced oxygen within the placental tissue can produce and release cytotoxic factors, which may affect the maternal blood supply during gestation. Inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), known to induce dysfunction in endothelial cells, are synthesized and released in the human placenta of patients with toxemia of pregnancy.
Recent reports indicate that hypoxia increases the production of TNF-α and IL-1 from the human placenta. Placental cells also express erythropoietin (EPO), a molecule regulated transcriptionally by hypoxia in mammals (25,28). Intriguingly, TNF-α and IL-1 exhibit DNA sequences homologous to the hypoxia-responsive enhancer element of the EPO gene, suggesting a potential, albeit untested, molecular link between placental hypoxia and the stimulation of cytokine production. Inflammatory cytokines excessively produced by the placenta in response to hypoxia may lead to their elevated plasma levels and induce endothelial activation and dysfunction in preeclampsia.
.PNG) Figure 4. TNF-α cascade |
Evidence-based research
A prospective case-control study was conducted at Zagazig University Hospitals, where 40 cases were recruited from the antenatal outpatient clinics in the Obstetrics and Gynecology Department. The sample size was determined using open EPI with a 95% confidence interval and 80% study power. The participants were divided into two groups: a standard (Control) group of 20 pregnant women at a gestational age of 28–34 weeks and a preeclampsia group comprising 20 women diagnosed according to the American College of Obstetricians and Gynecologists (ACOG) guidelines. The preeclampsia cases were further classified as severe based on specified criteria (Systolic blood pressure greater than or equal to 160 and diastolic blood pressure greater than or equal to 110 mmHg and proteinuria ≥5 mg/24 hours (29-31).
Patients were monitored every four hours starting at 10:00 daily, considering the circadian rhythm. The preeclampsia group was managed using antihypertensive medication, antioxidants, and close follow-up for two weeks. Magnesium sulfate infusion was selectively used for cases of severe preeclampsia to prevent neurologic deficit. IL-17 serum concentration was measured after two weeks, following additional blood pressure assessments. In the preeclampsia group, the mean IL-17 value was 18.5 pg/mL, while in the control group, it was 4.3 pg/mL, indicating a statistically significant difference between the groups. The receiver operating characteristic (ROC) curve identified the optimal cutoff value for IL-17 in preeclampsia as 8.2 pg/mL, demonstrating a sensitivity of 100%, specificity of 80%, and accuracy of 89% (29-31).
One study showed an elevation in IL-17 levels in 34 pre-eclamptic patients compared to 35 healthy pregnant women; they attributed their findings to an exacerbation of the normal inflammatory response preceding birth in preeclamptic patients. This result shows a significant difference in IL-17 levels before and after treatment of preeclampsia, with a significant positive association with systolic blood pressure. This inflammatory mediator can be very valuable for both prognosis and follow-up of the disease (29-31). Another research study conducted a similar investigation to evaluate cytokines linked with T-helper 17 (IL-17, IL-21, IL-23, and TGF-β) during the third trimester of pregnancy. The study included three cohorts: 30 preeclamptic patients, 30 normotensive pregnant women, and 30 healthy individuals (29). They observed that the serum concentrations of IL-17 and TGF-β were significantly elevated in preeclamptic patients in comparison to both the normotensive and the healthy individuals (P<0.0001) (29-31). The findings indicated a twofold increase in the median concentrations of plasma TNF-α in women with preeclampsia compared to normal third-trimester pregnancy (P < 0.001) and in women with gestational hypertension (P < 0.04) (29-31).
Anti-inflammatory cytokines in toxemia of pregnancy
In toxemia of pregnancy, there is an elevation in placental cytokines (Such as pro-inflammatory cytokines) alongside a diminished secretion of cytokines, such as IL-10 and IL-4, which typically inhibit inflammation (11,32). The essential role of anti-inflammatory cytokines (IL-4 and IL-10) is pivotal for the proper functioning of T helper cells type-2 (Th2) and regulatory T cells (Tregs) in a successful pregnancy and smooth progression to delivery (24). They also serve as a crucial immune system modulator and immunomodulator, directly enhancing vascular health and facilitating effective cellular interactions at the maternal-fetal interface. Alterations in the levels of these cytokines may impact the operation of the major apoptotic pathway, thereby affecting the smooth progression of pregnancy and leading to pregnancy-associated complications such as toxemia of pregnancy.
There has been a reported decrease in IL-10 production in trophoblasts from patients with toxemia of pregnancy under hypoxic conditions (32). This observation suggests that the pre-eclamptic placenta responds to hypoxia by producing inadequate levels of IL-10 and IL-4. Consequently, this abnormal response may contribute to the increased production of inflammatory cytokines, thereby contributing to maternal intravascular disease. One study reported a negative association between blood pressure and circulating IL-10 levels. This correlation has been primarily observed in non-human primates. This may indicate an association between toxemia of pregnancy and decreased systemic IL-10 bioactivity, which is confirmed by some studies (33,34).
In another study, variations in IL-10 levels were observed, with an increase in the first and second trimesters and a decline in the third trimester of normal pregnancies (2). However, in the present study, the levels and expression of IL-4 and IL-10 were diminished in both sets of pre-eclamptic placental tissues, contrasting with an upregulation in control samples (3,5). Similar patterns were observed in maternal serum samples, with lower levels detected in preeclampsia compared to the control group. These findings suggest that in toxemia of pregnancy, IL-10 and IL-4 may not effectively suppress the pro-inflammatory cytokines, potentially leading to heightened inflammatory responses (33,35).
Chemokines
Chemokines are a family of low-molecular-weight chemotactic cytokines that regulate leukocyte migration through interactions with rhodopsin-like G protein-coupled transmembrane receptors. Chemokines have significant structural homology and overlapping functions and can often bind to more than one receptor (36-38). Chemokine receptors mediate multiple signaling pathways that regulate various cellular responses.
The most studied chemokines are CC (β-chemokines), CXC (α-chemokines), CX3C, and C subfamilies. The C group of chemokines has recently been described. It has at least two ligands (XCL) and lacks cysteines (38). Examples are lymphotactin/XCL1 and SCM-1β/XCL2, which bind XCR1 receptors. Lymphotactin is coded for on human chromosome 1 and attracts lymphocytes, not monocytes or neutrophils. XCR1+ cells depend on the growth factor FtL3 ligand, and more studies should be conducted to establish its potential roles in toxemia of pregnancy (34,38). The human CC chemokine group with no intervening amino acid includes at least 27 members (CCL), most encoded on human chromosome 17, and binds at least 10 receptors (CCR). CC chemokine targets include monocytes, T cells, dendritic cells, eosinophils, and NK cells (38).
A prospective case-control study was conducted at Zagazig University Hospitals, where 40 cases were recruited from the antenatal outpatient clinics in the Obstetrics and Gynecology Department. The sample size was determined using open EPI with a 95% confidence interval and 80% study power. The participants were divided into two groups: a standard (Control) group of 20 pregnant women at a gestational age of 28–34 weeks and a preeclampsia group comprising 20 women diagnosed according to the American College of Obstetricians and Gynecologists (ACOG) guidelines. The preeclampsia cases were further classified as severe based on specified criteria (Systolic blood pressure greater than or equal to 160 and diastolic blood pressure greater than or equal to 110 mmHg and proteinuria ≥5 mg/24 hours (29-31).
Patients were monitored every four hours starting at 10:00 daily, considering the circadian rhythm. The preeclampsia group was managed using antihypertensive medication, antioxidants, and close follow-up for two weeks. Magnesium sulfate infusion was selectively used for cases of severe preeclampsia to prevent neurologic deficit. IL-17 serum concentration was measured after two weeks, following additional blood pressure assessments. In the preeclampsia group, the mean IL-17 value was 18.5 pg/mL, while in the control group, it was 4.3 pg/mL, indicating a statistically significant difference between the groups. The receiver operating characteristic (ROC) curve identified the optimal cutoff value for IL-17 in preeclampsia as 8.2 pg/mL, demonstrating a sensitivity of 100%, specificity of 80%, and accuracy of 89% (29-31).
One study showed an elevation in IL-17 levels in 34 pre-eclamptic patients compared to 35 healthy pregnant women; they attributed their findings to an exacerbation of the normal inflammatory response preceding birth in preeclamptic patients. This result shows a significant difference in IL-17 levels before and after treatment of preeclampsia, with a significant positive association with systolic blood pressure. This inflammatory mediator can be very valuable for both prognosis and follow-up of the disease (29-31). Another research study conducted a similar investigation to evaluate cytokines linked with T-helper 17 (IL-17, IL-21, IL-23, and TGF-β) during the third trimester of pregnancy. The study included three cohorts: 30 preeclamptic patients, 30 normotensive pregnant women, and 30 healthy individuals (29). They observed that the serum concentrations of IL-17 and TGF-β were significantly elevated in preeclamptic patients in comparison to both the normotensive and the healthy individuals (P<0.0001) (29-31). The findings indicated a twofold increase in the median concentrations of plasma TNF-α in women with preeclampsia compared to normal third-trimester pregnancy (P < 0.001) and in women with gestational hypertension (P < 0.04) (29-31).
Anti-inflammatory cytokines in toxemia of pregnancy
In toxemia of pregnancy, there is an elevation in placental cytokines (Such as pro-inflammatory cytokines) alongside a diminished secretion of cytokines, such as IL-10 and IL-4, which typically inhibit inflammation (11,32). The essential role of anti-inflammatory cytokines (IL-4 and IL-10) is pivotal for the proper functioning of T helper cells type-2 (Th2) and regulatory T cells (Tregs) in a successful pregnancy and smooth progression to delivery (24). They also serve as a crucial immune system modulator and immunomodulator, directly enhancing vascular health and facilitating effective cellular interactions at the maternal-fetal interface. Alterations in the levels of these cytokines may impact the operation of the major apoptotic pathway, thereby affecting the smooth progression of pregnancy and leading to pregnancy-associated complications such as toxemia of pregnancy.
There has been a reported decrease in IL-10 production in trophoblasts from patients with toxemia of pregnancy under hypoxic conditions (32). This observation suggests that the pre-eclamptic placenta responds to hypoxia by producing inadequate levels of IL-10 and IL-4. Consequently, this abnormal response may contribute to the increased production of inflammatory cytokines, thereby contributing to maternal intravascular disease. One study reported a negative association between blood pressure and circulating IL-10 levels. This correlation has been primarily observed in non-human primates. This may indicate an association between toxemia of pregnancy and decreased systemic IL-10 bioactivity, which is confirmed by some studies (33,34).
In another study, variations in IL-10 levels were observed, with an increase in the first and second trimesters and a decline in the third trimester of normal pregnancies (2). However, in the present study, the levels and expression of IL-4 and IL-10 were diminished in both sets of pre-eclamptic placental tissues, contrasting with an upregulation in control samples (3,5). Similar patterns were observed in maternal serum samples, with lower levels detected in preeclampsia compared to the control group. These findings suggest that in toxemia of pregnancy, IL-10 and IL-4 may not effectively suppress the pro-inflammatory cytokines, potentially leading to heightened inflammatory responses (33,35).
Chemokines
Chemokines are a family of low-molecular-weight chemotactic cytokines that regulate leukocyte migration through interactions with rhodopsin-like G protein-coupled transmembrane receptors. Chemokines have significant structural homology and overlapping functions and can often bind to more than one receptor (36-38). Chemokine receptors mediate multiple signaling pathways that regulate various cellular responses.
The most studied chemokines are CC (β-chemokines), CXC (α-chemokines), CX3C, and C subfamilies. The C group of chemokines has recently been described. It has at least two ligands (XCL) and lacks cysteines (38). Examples are lymphotactin/XCL1 and SCM-1β/XCL2, which bind XCR1 receptors. Lymphotactin is coded for on human chromosome 1 and attracts lymphocytes, not monocytes or neutrophils. XCR1+ cells depend on the growth factor FtL3 ligand, and more studies should be conducted to establish its potential roles in toxemia of pregnancy (34,38). The human CC chemokine group with no intervening amino acid includes at least 27 members (CCL), most encoded on human chromosome 17, and binds at least 10 receptors (CCR). CC chemokine targets include monocytes, T cells, dendritic cells, eosinophils, and NK cells (38).
|
Table 1. Summary of common cytokines and their function
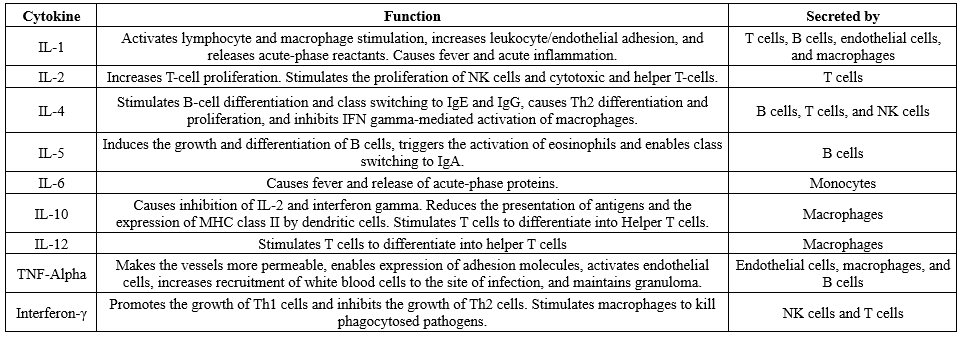 |
IL-8/CXCL8 (ELR), monokine-induced by IFN-γ (MIG)/CXCL9 (nonELR), IFN-γ inducible protein-10 (IP-10)/CXCL10 (nonELR), and stromal cell-derived factor-1 (SDF-1)/CXCL12 (nonELR) can be theoretically inferred to play a role in the pathogenesis of inflammatory changes of the placenta. Lastly, the “sole CX3C chemokine” (Three intervening amino acids), namely fractalkine/CX3CL1, is encoded on human chromosome 16, binds CX3CR1 and attracts T cells and monocytes but not neutrophils (38). Fractalkine/CX3CL1 is found in humans and might play a role in the neurological manifestations of toxemia during pregnancy.
A study of 309 pregnant women in three clusters (Uncomplicated preeclampsia with normal and abnormal angiogenic profiles) confirmed intravascular inflammation, and high cytokines and chemokines levels among the study groups. This study revealed that plasma concentrations of cytokines (Interleukin-6, interleukin-8, interleukin-12/interleukin-23p40, interleukin-15, and interleukin-16) and chemokines (eotaxin, eotaxin-3, interferon-γ inducible protein-10, monocyte chemotactic protein-4, macrophage inflammatory protein-1β, and macrophage-derived chemokine) were higher in pre-eclamptic women compared to the control group. However, except in preeclampsia, an average angiogenic profile where monocyte chemotactic protein 4 was the only elevated chemokine, a correlation was observed between the severity of the antiangiogenic state, blood pressure, and plasma concentrations of a subset of cytokines (35).
Conclusion
This study investigated the role of cytokines such as IL-6, IL-4, IL-10, TNF-α, and endothelin in the pathophysiology of pregnancy toxemia. There is evidence that lower levels of maternal IL-10 concentrations in the second trimester can predict pre-eclampsia. Clinical and animal studies on the role of cytokines in pregnancy toxemia have led to a broader understanding of its pathogenesis. Despite extensive research, the exact mechanism of pre-eclampsia remains unclear, necessitating further investigation.
Expanding knowledge in this area can facilitate accurate diagnoses and appropriate management plans. A greater understanding of chemokines and cytokines in pregnancy toxemia can prevent obstetric complications and enhance management and follow-up.
Abbreviations
Th, Helper T cell; RNA, Ribonucleic acid; DNA, Deoxyribonucleic acid; IL-1, Interleukins; ATP, Adenosine triphosphate; ROS, Reactive oxygen species; GM-CSF, Granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-γ; CXCL, chemokine (C-X-C motif) ligand; Chemokine (C-C motif) ligand; TLR, Toll-like receptor; DAMPS, Damage-associated molecular pattern; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; TNF, Tumor necrosis factor; TGF-β, Transforming growth factor-β; PIGF, Placental growth factor; VEGF, Vascular endothelial growth factor; TGF, Transforming growth factors; sFlt, Soluble fms-like tyrosine kinase-1; NO, Nitous oxide; RAAS, renin–angiotensin–aldosterone system; ANG, angiotensin; LFT, Liver function tests; PRES, posterior reversible encephalopathy syndrome.
Acknowledgement
The authors sincerely thank everyone who contributed to this research.
Funding sources
None.
Ethical statement
There are no ethical concerns regarding the article.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
All authors contributed to the article and approved the final version. CK created the diagrams and figures.
Data availability statement
The data and information backing the conclusions will be provided without any restrictions.
A study of 309 pregnant women in three clusters (Uncomplicated preeclampsia with normal and abnormal angiogenic profiles) confirmed intravascular inflammation, and high cytokines and chemokines levels among the study groups. This study revealed that plasma concentrations of cytokines (Interleukin-6, interleukin-8, interleukin-12/interleukin-23p40, interleukin-15, and interleukin-16) and chemokines (eotaxin, eotaxin-3, interferon-γ inducible protein-10, monocyte chemotactic protein-4, macrophage inflammatory protein-1β, and macrophage-derived chemokine) were higher in pre-eclamptic women compared to the control group. However, except in preeclampsia, an average angiogenic profile where monocyte chemotactic protein 4 was the only elevated chemokine, a correlation was observed between the severity of the antiangiogenic state, blood pressure, and plasma concentrations of a subset of cytokines (35).
Conclusion
This study investigated the role of cytokines such as IL-6, IL-4, IL-10, TNF-α, and endothelin in the pathophysiology of pregnancy toxemia. There is evidence that lower levels of maternal IL-10 concentrations in the second trimester can predict pre-eclampsia. Clinical and animal studies on the role of cytokines in pregnancy toxemia have led to a broader understanding of its pathogenesis. Despite extensive research, the exact mechanism of pre-eclampsia remains unclear, necessitating further investigation.
Expanding knowledge in this area can facilitate accurate diagnoses and appropriate management plans. A greater understanding of chemokines and cytokines in pregnancy toxemia can prevent obstetric complications and enhance management and follow-up.
Abbreviations
Th, Helper T cell; RNA, Ribonucleic acid; DNA, Deoxyribonucleic acid; IL-1, Interleukins; ATP, Adenosine triphosphate; ROS, Reactive oxygen species; GM-CSF, Granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-γ; CXCL, chemokine (C-X-C motif) ligand; Chemokine (C-C motif) ligand; TLR, Toll-like receptor; DAMPS, Damage-associated molecular pattern; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; TNF, Tumor necrosis factor; TGF-β, Transforming growth factor-β; PIGF, Placental growth factor; VEGF, Vascular endothelial growth factor; TGF, Transforming growth factors; sFlt, Soluble fms-like tyrosine kinase-1; NO, Nitous oxide; RAAS, renin–angiotensin–aldosterone system; ANG, angiotensin; LFT, Liver function tests; PRES, posterior reversible encephalopathy syndrome.
Acknowledgement
The authors sincerely thank everyone who contributed to this research.
Funding sources
None.
Ethical statement
There are no ethical concerns regarding the article.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
All authors contributed to the article and approved the final version. CK created the diagrams and figures.
Data availability statement
The data and information backing the conclusions will be provided without any restrictions.
Research Article: Review Article |
Subject:
Pathology
Received: 2024/05/9 | Accepted: 2025/02/1 | Published: 2025/02/11 | ePublished: 2025/02/11
Received: 2024/05/9 | Accepted: 2025/02/1 | Published: 2025/02/11 | ePublished: 2025/02/11
References
1. Dong C, Della-Morte D, Rundek T, Wright CB, Elkind MSV, Sacco RL. Evidence to Maintain the Systolic Blood Pressure Treatment Threshold at 140 mm Hg for Stroke Prevention. Hypertension. 2016;67(3):520-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Keelan JA, Mitchell MD. Placental cytokines and preeclampsia. Front Biosci. 2007;12(7):2706-27 [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Boraschi D, Penton-Rol G, Amodu O, Blomberg MT. Editorial: Women in Cytokines and Soluble Mediators in Immunity. Front Immunol. 2024:15:1395165. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Gathiram P, Moodley J. Pre-eclampsia: its pathogenesis and pathophysiology. Cardiovasc J Afr. 2016;27(2):71-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ Res.2019;124(7):1094-112. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. VADHERA RB, Simon M. Hypertensive Emergencies in Pregnancy. Clin Obstet Gynecol. 2014;57(4):797-805. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Titus A, Marappa-Ganeshan R. Physiology, Endothelin. PubMed. StatPearls. 2023. [View at Publisher] [PMID] [Google Scholar]
8. Ahmad S, Ahmed A. Elevated Placental Soluble Vascular Endothelial Growth Factor Receptor-1 Inhibits Angiogenesis in Preeclampsia. Circ Res. 2004;95(9):884-91. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Gohar EY, Pollock DM. Sex-Specific Contributions of Endothelin to Hypertension. 2018;20(7):58. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Bellos I, Karageorgiou V, Kapnias D, Karamanli KE, Siristatidis C. The Role of Interleukins in Preeclampsia: A Comprehensive Review. Am J Reprod Immunol. 2018;80(6):e13055 [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Tranquilli AL, Landi B, Corradetti A, Giannubilo SR, Sartini D, Pozzi V, et al. Inflammatory Cytokines Patterns in the placenta of Pregnancies Complicated by HELLP (hemolysis, elevated liver enzyme, and low platelet) syndrome. Cytokine. 2007;40(2):82-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Sargent IL, Borzychowski AM, Redman CWG. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online. 2006;13(5):680-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Huang X, Huang H, Dong M, Yao Q, Wang H. Serum and Placental Interleukin-18 Are Elevated in Preeclampsia. J Reprod Immunol. 2005;65(1):77-87. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Benyo DF, Miles TM, Conrad KP. Hypoxia Stimulates Cytokine Production by Villous Explants from the Human placenta. J Clin Endocrinol Metab.. 1997;82(5):1582-8 [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Sakai M, Tsuda H, Tanabe K, Sasaki Y, Saito S. Interleukin-12 Secretion by Peripheral Blood Mononuclear Cells Is Decreased in Normal Pregnant Subjects and Increased in Preeclamptic Patients. Am J Reprod Immunol. 2002;47(2):91-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Saito S, Umekage H, Sakamoto Y, Sakai M, Tanabe K, Sasaki Y, et al. Increased T-Helper-1-Type Immunity and Decreased T-Helper-2-Type Immunity in Patients with Preeclampsia. Am J Reprod Immunol. 1999;41(5):297-306. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Huang SJ, Zenclussen AC, Chen CP, Basar M, Yang H, Arcuri F, et al. The Implication of Aberrant GM-CSF Expression in Decidual Cells in the Pathogenesis of Preeclampsia. Am J Pathol. 2010;177(5):2472-82. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Preeclampsia." NHS Choices, NHS, www.nhs.uk/conditions/pre-eclampsia/. Accessed February 14, 2024. [View at Publisher] [Google Scholar]
19. Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, et al. Regulation of Monocyte Chemoattractant Protein-1 Expression by Tumor Necrosis Factor-α and Interleukin-1β in First Trimester Human Decidual Cells: implications for preeclampsia.Am J Pathol. 2006;168(2):445-52 [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995-1001. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Raghupathy R. Cytokines as Key Players in the Pathophysiology of Preeclampsia. Med Princ Pract, . 2013;22 Suppl 1(Suppl 1):8-19 [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, et al. Proportion of Peripheral Blood and Decidual CD4(+) Cd25(Bright) Regulatory T Cells in Preeclampsia. Clin Exp Immunol. 2007;149(1):139-45. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Bellos I, Karageorgiou V, Kapnias D, Karamanli KE, Siristatidis C. The role of interleukins in pre-eclampsia: A comprehensive review. Am J Reprod Immunol. 2018;80(6):e13055. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6, and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58(1):21-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of Tumor Necrosis Factor-α from Human Placental Tissues Induced by Hypoxia-Reoxygenation Causes Endothelial Cell Activation in Vitro. Am J Pathol. 2004;164(3):1049-61. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Vince GS, STARKEY PM, AUSTGULEN R, KWIATKOWSKI D, REDMAN CW. Interleukin-6, tumor necrosis factor and soluble tumor necrosis factor receptors in women with preeclampsia. Br J Obstet Gynaecol. 1995;102(1):20-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Presicce P, Cappelletti M, Senthamaraikannan P, Ma F, Morselli M, Jackson CM, et al. TNF-Signaling Modulates Neutrophil-Mediated Immunity at the Feto-Maternal Interface During LPS-Induced Intrauterine Inflammation. Front Immunol. 2020:11:558. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Benyo DF, Miles TM, Conrad KP. Hypoxia Stimulates Cytokine Production by Villous Explants from the Human Placenta. J Clin Endocrinol Metab. 1997;82(5):1582-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. El Shahaway AA, Abd Elhady RR, Abdelrhman AA, Yahia S. Role of maternal serum interleukin 17 in preeclampsia: diagnosis and prognosis. J Inflamm Res. 2019:12:175-80. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Darmochwal-Kolarz D, Michalak M, Kolarz B, Przegalinska-Kalamucka M ,Bojarska-Junak A, Sliwa D, et al. The Role of Interleukin-17, Interleukin-23, and Transforming Growth Factor-β in Pregnancy Complicated by Placental Insufficiency. Biomed Res Int. 2017:2017:6904325 [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Baharlou R, Atashzar MR, Ahmadi Vasmehjan A, Rahimi E, Khoshmirsafa M, Seif F. et al. Reduced Levels of T-Helper 17-Associated Cytokines in the Serum of Patients with Breast Cancer: Indicators for Following the Course of Disease. Cent Eur J Immunol. 2016;41(1):78-85. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Cubro H, Kashyap S, Nath MC, Ackerman AW, Garovic VD. The Role of Interleukin-10 in the Pathophysiology of Preeclampsia. Curr Hypertens Rep.2018;20(4):36. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Hashii K, Fujiwara H, Yoshioka S, Kataoka N, Yamada S, Hirano T, et al. Peripheral Blood Mononuclear Cells Stimulate Progesterone Production by Luteal Cells Derived from Pregnant and Non-Pregnant Women: Possible Involvement of Interleukin-4 and Interleukin-10 in Corpus Luteum Function and Differentiation. Hum Reprod. 1998;13(1O):2738-44. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. CHAIWORAPONGSA T, ROMERO R, GOMEZ-LOPEZ N, SUKSAI M, GALLO DM, JUNG E, et al. Preeclampsia at term: evidence of disease heterogeneity based on the profile of circulating cytokines and angiogenic factors. Am J Obstet Gynecol. 2024;230(4):450.e1-450.e18 [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97(2):540-50. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Romanowska-Próchnicka K, Felis-Giemza A, Olesińska M, Wojdasiewicz P, Paradowska-Gorycka A, Szukiewicz D . The Role of TNF-α and Anti-TNF-α Agents during Preconception, Pregnancy, and Breastfeeding. Int J Mol Sci. 2021;22(6):2922. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Mottola MF. Components of Exercise Prescription and Pregnancy. Clin Obstet Gynecol. 2016;59(3):552-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
38. Cameron MJ, Kelvin DJ. Cytokines, Chemokines and Their Receptors. Nih.gov. Landes Bioscience;2000-2013. [View at Publisher] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com