Volume 19, Issue 4 (Jul-Aug 2025)
mljgoums 2025, 19(4): 14-17 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sarkar S, Chavan S, Agrawal G, Rahangdale H, Zodpey S. Burkholderia cepacia complex: An outbreak in the neonatal intensive care unit of a tertiary care hospital in central India. mljgoums 2025; 19 (4) :14-17
URL: http://mlj.goums.ac.ir/article-1-1822-en.html
URL: http://mlj.goums.ac.ir/article-1-1822-en.html
1- Department of Microbiology, Goverment Medical College and Hospital (GMCH), Nagpur, Maharashtra, India , drshayosree.sarkar@gmail.com
2- Department of Microbiology, Goverment Medical College and Hospital (GMCH), Nagpur, Maharashtra, India
2- Department of Microbiology, Goverment Medical College and Hospital (GMCH), Nagpur, Maharashtra, India
Full-Text [PDF 495 kb]
(1144 Downloads)
| Abstract (HTML) (4500 Views)
Environmental surveillance was undertaken to identify the origin and transmission pathways of the infection. Samples were systematically collected from a variety of sources, including tap water, intravenous (IV) fluids (Fresh and opened), distilled water designated for humidifiers, water used in patient feeds, and antiseptic solutions. Additionally, pre-moistened sterile swabs were utilized to obtain samples from environmental surfaces, such as medicine trolleys, Ambu bags, and infant cradles (Table 1).
Samples were cultured on blood agar and MacConkey agar plates, which were then incubated at 37°C for 48 hours, as previously described (9). Tap water samples underwent centrifugation at 3000 rpm for 15 minutes, with the resulting sediment subsequently processed. All liquid samples were directly inoculated onto blood agar and MacConkey agar, as well as into brain-heart infusion broth (1:5 dilution). Bacterial plates underwent incubation at 37°C for a 48-hour period. Concurrently, the brain-heart infusion medium was incubated at the same temperature for 5 days, with daily assessments for turbidity (10). If turbidity was observed prior to the fifth day, or on the fifth day itself, subcultures were prepared from the brain-heart infusion and streaked onto blood agar and MacConkey agar plates. Environmental isolates were definitively identified using the automated VITEK 2 Compact microbiology analyser (BioMérieux, France).
For the purposes of this study, an outbreak was rigorously defined as the concurrent identification of more than two patients exhibiting positive culture results for BCC. Outbreak cases specifically referred to neonates who presented with clinical indicators suggestive of sepsis (Including fever, tachycardia, tachypnoea, leukocytosis, or leukopenia, with or without accompanying hypotension) and who also had at least one BCC-positive blood culture.
Results
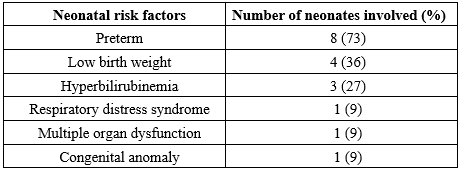
Eleven neonates with a clinical suspicion of sepsis all tested positive for BCC bacteremia. As detailed in Table 2, the predominant neonatal risk factor linked to this infection was preterm birth (73%), followed by low birth weight (LBW) (36%). The earliest gestational age recorded among the affected neonates was 28 weeks, and the minimum LBW observed was 1.6 kg.
Full-Text: (800 Views)
Introduction
The Burkholderia cepacia complex (BCC), a collection of gram-negative, non-fermenting, aerobic betaproteobacteria, was first identified in the 1980s as an opportunistic pathogen capable of causing severe and life-threatening infections in humans. This bacterial group employs a range of virulence mechanisms, contributing to its pathogenicity. These mechanisms include multidrug resistance (Via the bcrA efflux pump), genes that determine transmissibility (Such as esmR and cblA), siderophores (Including salicylic acid, ornibactin, pyochelin, and cepabactin), and adherence proteins (Notably long flexible type II pili) (1,2). These microorganisms exhibit considerable resilience to disinfection and sterilisation methods, demonstrating moderate to high tolerance to various stressors, including ultraviolet (UV)-C radiation, antibiotics, and elevated heavy-metal concentrations. Consequently, developing effective antimicrobial therapies against them presents a significant challenge. The United States Food and Drug Administration (FDA) has, thus, suggested classifying these bacteria as "Objectionable Microorganisms" (3). Critically ill neonates in low-resource healthcare settings are at an increased risk of acquiring nosocomial infections within neonatal units. This heightened vulnerability is largely attributed to suboptimal clinical care practices. Neonatal sepsis presents with a range of systemic indicators, such as fever, tachycardia, tachypnoea, and hypotension. A definitive diagnosis also requires the isolation of a pure bacterial culture from at least one anatomical site (4).
This article presents a detailed investigation into a BCC outbreak that affected neonates within a neonatal intensive care unit (NICU) over a one-month period. The successful identification of the outbreak's source was critically dependent on the active involvement of the hospital's infection control team. Subsequently, timely and precisely targeted interventions, derived from these crucial findings, proved indispensable in effectively containing and controlling the spread of the infection.
Methods
This cross-sectional study, conducted in July 2023 at a tertiary care hospital, investigated 11 neonates referred from the NICU with clinical suspicion of sepsis. All referrals were for blood culture investigations. Biological parameters indicative of infection were recorded, including elevated C-reactive protein (CRP) levels (≥6 mg/dL), along with observations of leucocytosis and thrombocytopenia. Blood samples submitted for routine diagnostic testing were processed by the Department of Microbiology at Government Medical College, Nagpur, India. Conventional blood culture bottles (5), specifically five containing brain-heart infusion broth supplemented with 0.025% sodium polyanethol sulfonate, were utilized. Following standard protocols, these blood culture samples underwent sub-culturing onto blood agar, chocolate agar, and MacConkey agar (6). Bacterial cultures grown on blood agar exhibited characteristic smooth, grey, and translucent colonies. Concurrently, non-lactose fermenting (NLF) colonies were observed on MacConkey's agar (Figure 1). Gram-negative isolates demonstrating motility and positive reactions for both catalase and oxidase were subsequently identified to the species level through conventional biochemical testing, as detailed in Figure 2 (6,7). The identification of these isolates was subsequently corroborated using the automated VITEK 2 Compact microbiology analyser (BioMérieux, France). Antimicrobial susceptibility was assessed using both the modified Kirby-Bauer disk diffusion method and the minimum inhibitory concentration (MIC) by the VITEK 2 antimicrobial susceptibility testing (AST) card. Both methodologies adhered to the guidelines established by the Clinical and Laboratory Standards Institute (2023) (8). All essential media, biochemicals, and disks required for AST procedures were sourced from HiMedia, India.
The Burkholderia cepacia complex (BCC), a collection of gram-negative, non-fermenting, aerobic betaproteobacteria, was first identified in the 1980s as an opportunistic pathogen capable of causing severe and life-threatening infections in humans. This bacterial group employs a range of virulence mechanisms, contributing to its pathogenicity. These mechanisms include multidrug resistance (Via the bcrA efflux pump), genes that determine transmissibility (Such as esmR and cblA), siderophores (Including salicylic acid, ornibactin, pyochelin, and cepabactin), and adherence proteins (Notably long flexible type II pili) (1,2). These microorganisms exhibit considerable resilience to disinfection and sterilisation methods, demonstrating moderate to high tolerance to various stressors, including ultraviolet (UV)-C radiation, antibiotics, and elevated heavy-metal concentrations. Consequently, developing effective antimicrobial therapies against them presents a significant challenge. The United States Food and Drug Administration (FDA) has, thus, suggested classifying these bacteria as "Objectionable Microorganisms" (3). Critically ill neonates in low-resource healthcare settings are at an increased risk of acquiring nosocomial infections within neonatal units. This heightened vulnerability is largely attributed to suboptimal clinical care practices. Neonatal sepsis presents with a range of systemic indicators, such as fever, tachycardia, tachypnoea, and hypotension. A definitive diagnosis also requires the isolation of a pure bacterial culture from at least one anatomical site (4).
This article presents a detailed investigation into a BCC outbreak that affected neonates within a neonatal intensive care unit (NICU) over a one-month period. The successful identification of the outbreak's source was critically dependent on the active involvement of the hospital's infection control team. Subsequently, timely and precisely targeted interventions, derived from these crucial findings, proved indispensable in effectively containing and controlling the spread of the infection.
Methods
This cross-sectional study, conducted in July 2023 at a tertiary care hospital, investigated 11 neonates referred from the NICU with clinical suspicion of sepsis. All referrals were for blood culture investigations. Biological parameters indicative of infection were recorded, including elevated C-reactive protein (CRP) levels (≥6 mg/dL), along with observations of leucocytosis and thrombocytopenia. Blood samples submitted for routine diagnostic testing were processed by the Department of Microbiology at Government Medical College, Nagpur, India. Conventional blood culture bottles (5), specifically five containing brain-heart infusion broth supplemented with 0.025% sodium polyanethol sulfonate, were utilized. Following standard protocols, these blood culture samples underwent sub-culturing onto blood agar, chocolate agar, and MacConkey agar (6). Bacterial cultures grown on blood agar exhibited characteristic smooth, grey, and translucent colonies. Concurrently, non-lactose fermenting (NLF) colonies were observed on MacConkey's agar (Figure 1). Gram-negative isolates demonstrating motility and positive reactions for both catalase and oxidase were subsequently identified to the species level through conventional biochemical testing, as detailed in Figure 2 (6,7). The identification of these isolates was subsequently corroborated using the automated VITEK 2 Compact microbiology analyser (BioMérieux, France). Antimicrobial susceptibility was assessed using both the modified Kirby-Bauer disk diffusion method and the minimum inhibitory concentration (MIC) by the VITEK 2 antimicrobial susceptibility testing (AST) card. Both methodologies adhered to the guidelines established by the Clinical and Laboratory Standards Institute (2023) (8). All essential media, biochemicals, and disks required for AST procedures were sourced from HiMedia, India.
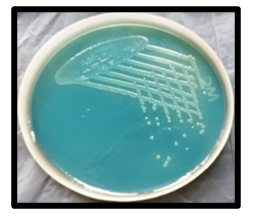 Figure 1. Non-lactose fermenting translucent moist colonies on MacConkey’s agar  Figure 2. Biochemical results: Triple Sugar Iron (TSI): K/K, with no gas or H2S production; citrate utilized; urea not hydrolyzed; indole negative; methyl red negative; base without any added amino acids, indicating the viability of the organism; ornithine not decarboxylated; lysine decarboxylated; arginine hydrolyzed. |
Environmental surveillance was undertaken to identify the origin and transmission pathways of the infection. Samples were systematically collected from a variety of sources, including tap water, intravenous (IV) fluids (Fresh and opened), distilled water designated for humidifiers, water used in patient feeds, and antiseptic solutions. Additionally, pre-moistened sterile swabs were utilized to obtain samples from environmental surfaces, such as medicine trolleys, Ambu bags, and infant cradles (Table 1).
Samples were cultured on blood agar and MacConkey agar plates, which were then incubated at 37°C for 48 hours, as previously described (9). Tap water samples underwent centrifugation at 3000 rpm for 15 minutes, with the resulting sediment subsequently processed. All liquid samples were directly inoculated onto blood agar and MacConkey agar, as well as into brain-heart infusion broth (1:5 dilution). Bacterial plates underwent incubation at 37°C for a 48-hour period. Concurrently, the brain-heart infusion medium was incubated at the same temperature for 5 days, with daily assessments for turbidity (10). If turbidity was observed prior to the fifth day, or on the fifth day itself, subcultures were prepared from the brain-heart infusion and streaked onto blood agar and MacConkey agar plates. Environmental isolates were definitively identified using the automated VITEK 2 Compact microbiology analyser (BioMérieux, France).
For the purposes of this study, an outbreak was rigorously defined as the concurrent identification of more than two patients exhibiting positive culture results for BCC. Outbreak cases specifically referred to neonates who presented with clinical indicators suggestive of sepsis (Including fever, tachycardia, tachypnoea, leukocytosis, or leukopenia, with or without accompanying hypotension) and who also had at least one BCC-positive blood culture.
|
Table 1. Swabs collected for environmental surveillance
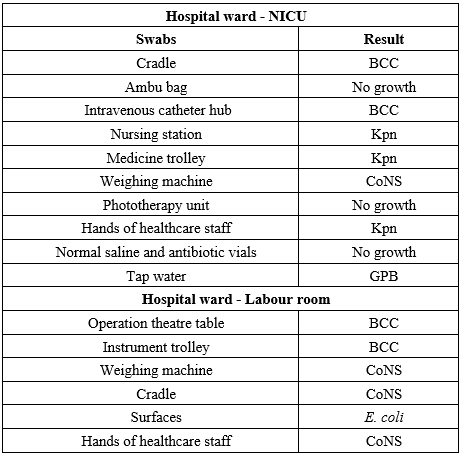 Abbreviations: NICU: Neonatal Intensive Care Unit; BCC: Burkholderia Cepacia Complex; Kpn: Klebsiella pneumoniae; CoNS: Coagulase Negative Staphylococcus; GPB: Gram-Positive Bacilli; E. coli: Escherichia coli |
Results
Eleven neonates with a clinical suspicion of sepsis all tested positive for BCC bacteremia. As detailed in Table 2, the predominant neonatal risk factor linked to this infection was preterm birth (73%), followed by low birth weight (LBW) (36%). The earliest gestational age recorded among the affected neonates was 28 weeks, and the minimum LBW observed was 1.6 kg.
|
Table 2. Risk factors associated with Burkholderia cepacia complex infection
 |
Environmental monitoring has identified the sources of BCC contamination within the healthcare facility. As detailed in Table 1, these sources include IV catheters and patient cradles in the NICU, operating theatre (OT) tables, and instrument trolleys in the labour room.
Biochemical profiling and AST indicated a close relationship between the bacterial strain under investigation and isolates obtained from neonatal blood cultures. AST further demonstrated that all strains were susceptible to levofloxacin, cotrimoxazole, and minocycline. However, a small number of these strains exhibited resistance to meropenem and ceftazidime.
Discussion
In this study, all 11 neonates who tested positive for BCC in blood cultures were clinically diagnosed with sepsis. These cases presented with diverse pre-existing conditions, including preterm birth, LBW, respiratory distress syndrome (RDS), hyperbilirubinemia, and congenital anomalies (Tetralogy of Fallot). The most prevalent neonatal risk factors identified were preterm birth (Gestational age <37 weeks), followed by LBW (<2.5 kg), and hyperbilirubinemia. These observations align with findings from previous studies conducted by Belachew et al. (11) and Murthy et al. (12).
Many healthcare professionals administer antibiotics without prior culture of the infection site, which contributes to the nosocomial spread of multidrug-resistant organisms, such as BCC. While previous studies have indicated a higher susceptibility of these organisms to meropenem and ceftazidime (13,14), our study observed a considerably lower susceptibility. Analysis of isolate susceptibility revealed levofloxacin to be the most effective agent, demonstrating superior activity compared to co-trimoxazole and minocycline. Given this resistance profile, levofloxacin was identified as the optimal therapeutic choice. Consequently, following a thorough assessment of its risk-benefit ratio under close medical supervision, levofloxacin therapy was initiated.
Despite therapeutic interventions, all neonates exhibited persistent sepsis without any clinical improvement. Consequently, the antibiotic regimens were modified based on AST results. Following these adjustments, 7 patients demonstrated a positive response to treatment, whereas 4 neonates unfortunately succumbed. Among the fatalities, two were attributed to disseminated intravascular coagulation (DIC), with pre-existing LBW and RDS identified as antecedent contributing factors. The third infant's demise was attributed to septic shock. This individual was diagnosed with acyanotic congenital heart disease. The fourth infant succumbed to sepsis with DIC, with antecedent conditions including hypoxic-ischemic encephalopathy (HIE), preterm birth, and LBW.
Surveillance data indicated that BCC isolated from the surfaces of OT beds and instrument trolleys in the labour room may have contributed to the spread of infection during infant deliveries. Consequently, a direct correlation was identified between vaginal delivery and the occurrence of neonatal sepsis. This finding aligns with the research conducted by Pataskar et al. (15). The presence of environmental strains on the cradle surfaces may be attributed to insufficient aseptic techniques (Such as improper hand hygiene and inadequate surface disinfection), given that BCC is known to persist in nutrient-limited and moist hospital environments (16).
It was observed that every neonate had a peripheral IV catheter, which demonstrates a potential pathway for the dissemination of BCC. BCC members are ubiquitous in the environment and are known for their ability to persist and proliferate even in the presence of disinfectants and on indwelling invasive medical devices. Consequently, they can serve as a significant reservoir for infections, particularly in immunocompromised and hospitalized patients. There is growing recognition of BCC as a colonizer of contaminated medical equipment during hospitalization (17).
The total mortality rate could be reduced to just four cases through the implementation of a robust hospital infection control policy, encompassing (i) thorough documentation and clear communication of the outbreak status to both clinicians and administrators; (ii) rapid reporting by microbiologists, coupled with immediate responses from clinicians; (iii) strict adherence to safe injection practices; (iv) rigorous enforcement of infection prevention and control measures, including meticulous hand hygiene, comprehensive environmental cleaning, appropriate disinfectant use, and proper segregation of biomedical waste; and (v) regular competency-based training alongside continuous monitoring of adherence to these guidelines.
NICU-admitted patients frequently require multiple invasive medical devices, including peripheral and central IV catheters, urinary catheters, and invasive mechanical ventilation. Consequently, we advocate for obtaining multiple cultures from various anatomical sites of any NICU patient immediately upon admission and whenever new symptoms emerge. This approach successfully controlled the outbreak, with 7 out of 11 infants recovering and being discharged. No new cases of BCC were reported in the subsequent months.
Several studies on outbreak investigations have been documented in India. For instance, Bhise et al. reported a NICU outbreak in Nagpur, where 10 neonates tested positive for BCC bacteremia; however, the infection source remained unidentified (18). In another instance, Mali et al. detailed an outbreak investigation among pediatric patients, where the source of BCC was traced to the upper surface of rubber stoppers on sealed multidose amikacin vials (19).
This study underscores the critical role of environmental surveillance in effectively managing outbreaks of BCC among NICU-admitted patients.
Conclusion
The considerable risk of BCC as a prevalent nosocomial pathogen in neonates necessitates close scrutiny. This emphasizes the critical need for stringent monitoring, proper drug testing, and the implementation of a robust hospital infection control policy. The present research highlights the critical role of prompt and timely intervention by a hospital's infection control committee during an outbreak. It emphasizes that comprehensive diagnostic efforts and ongoing surveillance are indispensable for both confirming and effectively managing such events.
Acknowledgement
We would like to thank the faculty members and technical staff of the Microbiology Department for their invaluable assistance.
Funding sources
This study did not receive any grants from funding agencies in the public, commercial, or non-profit organizations.
Ethical statement
Institutional Ethics Committee (IES; Ref. No. EC/Pharmac/GMC/NGP/4120).
Conflicts of interest
No conflicts of interest.
Author contributions
Dr. Shayosree Sarkar and Dr. Sonal Chavan: Conceptualization, Methodology, and Manuscript preparation. Dr. Geetika Agrawal and Dr. Shayosree Sarkar: Sample acquisition. Dr. Heena Rahangdale: Critical review and Editing of the manuscript. Dr. Sunanda Zodpey (Shrikhande): Project supervision.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Biochemical profiling and AST indicated a close relationship between the bacterial strain under investigation and isolates obtained from neonatal blood cultures. AST further demonstrated that all strains were susceptible to levofloxacin, cotrimoxazole, and minocycline. However, a small number of these strains exhibited resistance to meropenem and ceftazidime.
Discussion
In this study, all 11 neonates who tested positive for BCC in blood cultures were clinically diagnosed with sepsis. These cases presented with diverse pre-existing conditions, including preterm birth, LBW, respiratory distress syndrome (RDS), hyperbilirubinemia, and congenital anomalies (Tetralogy of Fallot). The most prevalent neonatal risk factors identified were preterm birth (Gestational age <37 weeks), followed by LBW (<2.5 kg), and hyperbilirubinemia. These observations align with findings from previous studies conducted by Belachew et al. (11) and Murthy et al. (12).
Many healthcare professionals administer antibiotics without prior culture of the infection site, which contributes to the nosocomial spread of multidrug-resistant organisms, such as BCC. While previous studies have indicated a higher susceptibility of these organisms to meropenem and ceftazidime (13,14), our study observed a considerably lower susceptibility. Analysis of isolate susceptibility revealed levofloxacin to be the most effective agent, demonstrating superior activity compared to co-trimoxazole and minocycline. Given this resistance profile, levofloxacin was identified as the optimal therapeutic choice. Consequently, following a thorough assessment of its risk-benefit ratio under close medical supervision, levofloxacin therapy was initiated.
Despite therapeutic interventions, all neonates exhibited persistent sepsis without any clinical improvement. Consequently, the antibiotic regimens were modified based on AST results. Following these adjustments, 7 patients demonstrated a positive response to treatment, whereas 4 neonates unfortunately succumbed. Among the fatalities, two were attributed to disseminated intravascular coagulation (DIC), with pre-existing LBW and RDS identified as antecedent contributing factors. The third infant's demise was attributed to septic shock. This individual was diagnosed with acyanotic congenital heart disease. The fourth infant succumbed to sepsis with DIC, with antecedent conditions including hypoxic-ischemic encephalopathy (HIE), preterm birth, and LBW.
Surveillance data indicated that BCC isolated from the surfaces of OT beds and instrument trolleys in the labour room may have contributed to the spread of infection during infant deliveries. Consequently, a direct correlation was identified between vaginal delivery and the occurrence of neonatal sepsis. This finding aligns with the research conducted by Pataskar et al. (15). The presence of environmental strains on the cradle surfaces may be attributed to insufficient aseptic techniques (Such as improper hand hygiene and inadequate surface disinfection), given that BCC is known to persist in nutrient-limited and moist hospital environments (16).
It was observed that every neonate had a peripheral IV catheter, which demonstrates a potential pathway for the dissemination of BCC. BCC members are ubiquitous in the environment and are known for their ability to persist and proliferate even in the presence of disinfectants and on indwelling invasive medical devices. Consequently, they can serve as a significant reservoir for infections, particularly in immunocompromised and hospitalized patients. There is growing recognition of BCC as a colonizer of contaminated medical equipment during hospitalization (17).
The total mortality rate could be reduced to just four cases through the implementation of a robust hospital infection control policy, encompassing (i) thorough documentation and clear communication of the outbreak status to both clinicians and administrators; (ii) rapid reporting by microbiologists, coupled with immediate responses from clinicians; (iii) strict adherence to safe injection practices; (iv) rigorous enforcement of infection prevention and control measures, including meticulous hand hygiene, comprehensive environmental cleaning, appropriate disinfectant use, and proper segregation of biomedical waste; and (v) regular competency-based training alongside continuous monitoring of adherence to these guidelines.
NICU-admitted patients frequently require multiple invasive medical devices, including peripheral and central IV catheters, urinary catheters, and invasive mechanical ventilation. Consequently, we advocate for obtaining multiple cultures from various anatomical sites of any NICU patient immediately upon admission and whenever new symptoms emerge. This approach successfully controlled the outbreak, with 7 out of 11 infants recovering and being discharged. No new cases of BCC were reported in the subsequent months.
Several studies on outbreak investigations have been documented in India. For instance, Bhise et al. reported a NICU outbreak in Nagpur, where 10 neonates tested positive for BCC bacteremia; however, the infection source remained unidentified (18). In another instance, Mali et al. detailed an outbreak investigation among pediatric patients, where the source of BCC was traced to the upper surface of rubber stoppers on sealed multidose amikacin vials (19).
This study underscores the critical role of environmental surveillance in effectively managing outbreaks of BCC among NICU-admitted patients.
Conclusion
The considerable risk of BCC as a prevalent nosocomial pathogen in neonates necessitates close scrutiny. This emphasizes the critical need for stringent monitoring, proper drug testing, and the implementation of a robust hospital infection control policy. The present research highlights the critical role of prompt and timely intervention by a hospital's infection control committee during an outbreak. It emphasizes that comprehensive diagnostic efforts and ongoing surveillance are indispensable for both confirming and effectively managing such events.
Acknowledgement
We would like to thank the faculty members and technical staff of the Microbiology Department for their invaluable assistance.
Funding sources
This study did not receive any grants from funding agencies in the public, commercial, or non-profit organizations.
Ethical statement
Institutional Ethics Committee (IES; Ref. No. EC/Pharmac/GMC/NGP/4120).
Conflicts of interest
No conflicts of interest.
Author contributions
Dr. Shayosree Sarkar and Dr. Sonal Chavan: Conceptualization, Methodology, and Manuscript preparation. Dr. Geetika Agrawal and Dr. Shayosree Sarkar: Sample acquisition. Dr. Heena Rahangdale: Critical review and Editing of the manuscript. Dr. Sunanda Zodpey (Shrikhande): Project supervision.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Research Article: Original Paper |
Subject:
Virology
Received: 2024/06/12 | Accepted: 2025/03/17 | Published: 2025/09/17 | ePublished: 2025/09/17
Received: 2024/06/12 | Accepted: 2025/03/17 | Published: 2025/09/17 | ePublished: 2025/09/17
References
1. Depoorter E, Bull MJ, Peeters C, Coenye T, Vandamme P, Mahenthiralingam E. Burkholderia: an update on taxonomy and biotechnological potential as antibiotic producers. Appl Microbiol Biotechnol. 2016;100(12):5215-29. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Safdar A. Stenotrophomonas maltophilia and Burkholderia cepacia. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Elsevier. 2015;2532-2540.e4. [View at Publisher] [DOI]
3. Sutton S. What is an objectionable organism. Am Pharmaceut Rev. 2012;15(6):36-48. [View at Publisher]
4. Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365(9465):1175-88 [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Collee JG, Marr W. Culture containers and culture media. Mackie and McCartney Practical Microbiology. 14th ed., Ch. 6. New York: Churchill Livingstone; 1996;131-49. [View at Publisher]
6. Mackie TJ. Mackie and McCartney practical medical microbiology 14th edition. New York: Churchill Livingstone. 2006. [View at Publisher]
7. Procop GW, Church DL, Hall GS, Janda WM,Koneman EW,Schreckenberger PC, et al. Koneman's color atlas and textbook of diagnostic microbiology. Jones & Bartlett Learning; 2020:1. [View at Publisher] [Google Scholar]
8. Clinical and Laboratory Standards Institute (CLSI) document M100-Ed33. Performance standards for antimicrobial susceptibility testing. 33rd Inf Suppl. 2023;39(1):50-2. [View at Publisher]
9. Doit C, Loukil C, Simon AM, Ferroni A, Fontan JE, Bonacorsi S, et al. Outbreak of Burkholderia cepacia Bacteremia in a Pediatric Hospital Due to Contamination of Lipid Emulsion Stoppers. J Clin Microbiol. 2004;42(5):2227-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Nasser RM, Rahi AC, Haddad MF, Daoud Z, Irani-Hakime N, Almawi WY. Outbreak of Burkholderia Cepacia Bacteremia Traced to Contaminated Hospital Water Used for Dilution of an Alcohol Skin Antiseptic. Infect Control Hosp Epidemiol. 2004;25(3):231-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Belachew A, Tewabe T. Neonatal sepsis and its association with birth weight and gestational age among admitted neonates in Ethiopia: systematic review and meta-analysis. BMC Pediatrics. 2020;20(1):55. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Murthy S, Godinho MA, Guddattu V, Lewis LES, Nair NS. Risk factors of neonatal sepsis in India: A systematic review and meta-analysis. PloS One. 2019;14(4):e0215683. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Patra S, Bhat Y R, Lewis LE, Purakayastha J, Sivaramaraju VV, Kalwaje E V, et al. Burkholderia cepacia sepsis among neonates. Indian J Pediatr. 2014;81(11):1233-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. El Chakhtoura NG, Saade E, Wilson BM, Perez F, Papp-Wallace KM, Bonomo RA. A 17-Year Nationwide Study of Burkholderia cepacia Complex Bloodstream Infections Among Patients in the United States Veterans Health Administration.Clin Infect Dis. 2017;65(8):1253-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Pataskar A, Chandel A, Chauhan V, Jain M. Gram-negative Late Onset Neonatal Sepsis in a Tertiary Care Center From Central India: A Retrospective Analysis. Clin Med Insights Pediatr. 2023;17:11795565231189595. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Torbeck L, Raccasi D, Guilfoyle DE, Friedman RL, Hussong D. Burkholderia cepacia: This Decision Is Overdue. PDA J Pharm Sci Technol. 2011;65(5):535-43. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Bharara T, Chakravarti A, Sharma M, Agarwal P. Investigation of Burkholderia cepacia complex bacteremia outbreak in a neonatal intensive care unit: a case series. J Med Case Reports. 2020;14(1):76. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Bhise SM, Rahangdale VA, Qazi MS. Burkholderia Cepacia an emerging cause of septicemia-an outbreak in a neonatal Intensive Care Unit from a tertiary care hospital of central India. IOSR J Dent Med Sci. 2013;10:41-3. [View at Publisher] [DOI] [Google Scholar]
19. Mali S, Dash L, Gautam V, Shastri J, Kumar S. An Outbreak of Burkholderia cepacia Complex in the Paediatric Unit of a Tertiary Care Hospital. Indian J Med Microbiol. 2017;35(2):216-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com